DMSO/I2: Simple and Green Syntheses of Imidazoles
Imidazoles are crucial building blocks for many pharmaceuticals and agrichemicals. Trisubstituted imidazoles are particularly important. Therefore, several methods have been developed to synthesize these structural units; however, these methods generally have several drawbacks, including the need for complex and harsh reaction conditions and toxic transition metal reagents. Additionally, they sometimes produce many side products. Therefore, yields are often low. Two recent examples, however, demonstrate how the green DMSO/I2 reagent pair may be used to build these structural units.1-2
The iodine/DMSO pair works in the following fashion (Figure 1). Iodine reacts with a ketone 1 or an alkyne 2 at a labile position to produce intermediate A or B respectively. Then DMSO oxidizes that position to produce the diketone 3, and iodide ion(s) is(are) returned to the solution. DMSO oxidizes I– to I2, so in many cases DMSO/I2 reactions can be run under catalytic conditions. While this is not the case in the two examples below (one requiring 0.5 equiv of iodine and the other 1.5 equiv), they still do not require large excesses.
Figure 1: DMSO/I2 routes to a common diketone intermediate in the synthesis of imidazoles.
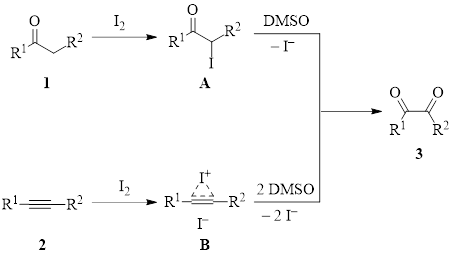
Once the diketone is formed, it is reactive to attack by nitrogen nucleophiles, leading to cyclization/condensation reactions which yield imidazoles.
The first of these reactions is shown in Figure 2, starting from ketone 1 which contains an α hydrogen (path a).1 The second begins with alkyne 2 (path b).2 They both result in the transient diketone intermediate 3. Addition of an aldehyde and ammonia then provides the three-component cyclization/condensation product of the imidazole 4. Good to excellent yields are provided in all cases where R1, R2, and R3 on the ketone, alkyne, and aldehyde are aromatic groups. In cases where R1 ≠ R2, a mixture of tautomers is produced, albeit in good yield. If R3 is aliphatic, the imidazole can be produced in some cases, but the yield is diminished.
Figure 2: DMSO/I2 synthesis of imidazoles from a ketone or an alkyne.
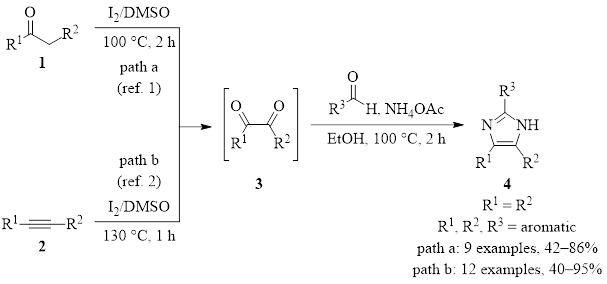
These examples add to the growing list of nitrogen heterocycles that can be synthesized with the DMSO/I2 pairing. For other examples, a recent review covers other nitrogen heterocycles that arise from similar pathways.3 Again, in these examples DMSO shows that it can play versatile roles beyond just being a solvent. As these reactions are efficient, eco-friendly, and inexpensive, it appears obvious that the number of DMSO/I2 transformations will only continue to grow.
Debra D. Dolliver, Ph.D.
References
- Jayram, J.; Jeena, V., An iodine/DMSO-catalyzed sequential one-pot approach to 2,4,5-trisubstituted-1H-imidazoles from α-methylene ketones. RSC Advances 2018, 8 (66), 37557-37563.
- Naidoo, S.; Jeena, V., One-Pot, Two-Step Metal and Acid-Free Synthesis of Trisubstituted Imidazole Derivatives via Oxidation of Internal Alkynes Using an Iodine/DMSO System. European Journal of Organic Chemistry 2019, 2019 (5), 1107-1113.
- Monga, A.; Bagchi, S.; Sharma, A., Iodine/DMSO oxidations: a contemporary paradigm in C–N bond chemistry. New Journal of Chemistry 2018, 42 (3), 1551-1576.







Leave a Reply
You must be logged in to post a comment.