DMSO-I2-Promoted Insertion of Isocyanide into the N–H Bond of Amines: Synthesis of Ureas
Metal-catalyzed insertion reactions of isocyanides into amines have been developed with the ultimate goal of synthesizing nitrogen-containing heterocycles. These routes have had problems in that isocyanides often strongly coordinate to metal catalysts, and they often undergo polymerization under these conditions.1
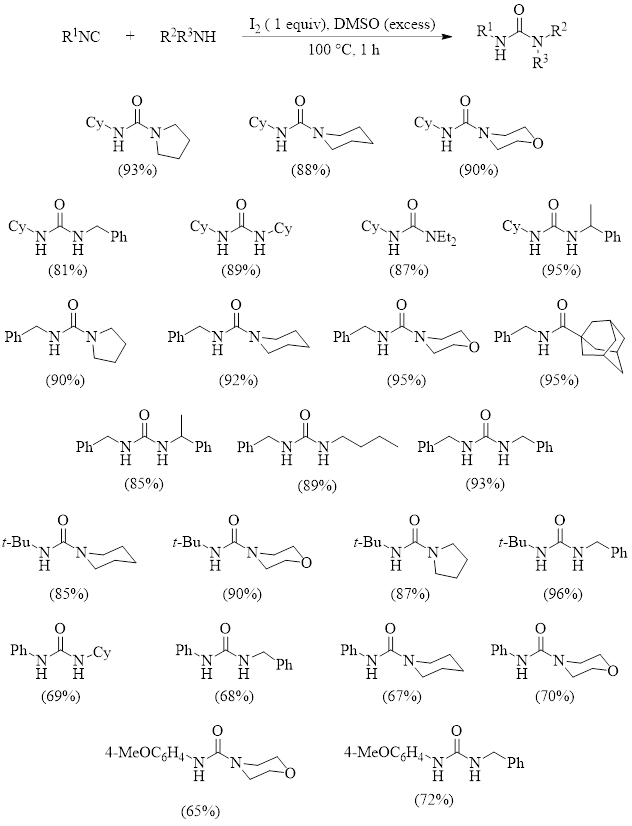
Because of this, there is interest in developing metal-free methods. The metal-free insertion of an isocyanide into the N–H bond is rare.2,3 This makes a 2018 report by Bora and Bez important, as it describes a new method for DMSO-I2-promoted isocyanide insertion into the N–H bond of an amine.4 This reaction results in various highly substituted ureas which have numerous applications in organic synthesis and pharmaceutical fields. Table 1 illustrates the substrate scope of this reaction when using the optimized conditions shown in the reaction at the top of the table.
As can be seen, this reaction tolerates aliphatic and aromatic isocyanides, affording good yields even when bulky aliphatic groups are incorporated into the isocyanide (R1 = t-Bu). The reaction also tolerates a variety of primary and secondary aliphatic amines. These researchers found that the reaction failed when using an aromatic amine (aniline) under these conditions. They discovered, however, that inclusion of 1.2 equivalents of DABCO to the reaction allowed for modest yields of ureas when aromatic amines are used.
Table 1: Optimized Conditions and Substrate Scope Studies
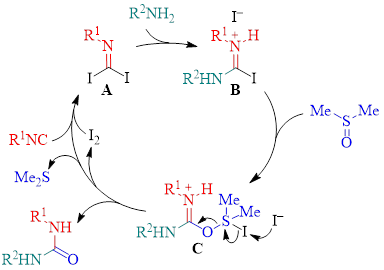
Work with 18O-labeled DMSO indicated that the 18O label is incorporated into the final urea product. This allowed the authors to propose the mechanism shown in Scheme 1. In this mechanism, the isocyanide reacts with I2 to make the activated isocyanide diiodide species A. Interaction of diiodide A with an amine leads to intermediate B. Dimethyl sulfoxide attacks and substitutes the iodide of intermediate B to make intermediate C. Subsequent loss of I2 (which then reenters the mechanism cycle) and dimethyl sulfide results in the formation of the product urea.
Scheme 1: Proposed Mechanism
This work demonstrates a high-yielding, metal-free route to symmetric and asymmetric ureas using green and readily-available I2 and DMSO. Iodine is thought to activate the isocyanide to nucleophilic attack, and DMSO acts as an oxidant in the reaction.
Debra D. Dolliver, Ph.D.
References:
- M. Suginome and Y. Ito, Transition Metal-Mediated Polymerization of Isocyanides, Polymer Synthesis, Advances in Polymer Science, Springer, Berlin, Heidelberg, 2004, vol. 272, pp. 77–136.
- H.-J. Ai, C.-X. Cai, X. Qi, J.-B. Peng, J. Ying, F. Zheng and X.-F. Wu, Tetrahedron Lett., 2017, 58, 3751.
- T.-H. Zhu, S.-Y. Wang, Y.-Q. Tao and S.-J. Ji, Org. Lett., 2015, 17, 1974.
- P. Bora and G. Bez, Chem. Commun., 2018, 54, 8363.

Leave a Reply
You must be logged in to post a comment.