DMSO as the carbon source for sp3 C-H olefination
The examples of dimethyl sulfoxide acting as a one carbon source for methylenation reactions continue to grow. (See recent Synthesis Corner posts from January, 2018—“DMSO as the carbon source in transition-metal-free α-Csp3-H methenylation” and March, 2018—“DMSO as a one carbon source in the transition-metal-free synthesis of quinolones.”) Recently, Namitharan et al. reported on the synthesis of multisubstituted arylamidines in a one-pot sequential approach using hypervalent aryliodide and DMSO to first perform an α sp3 C-H olefination followed by a palladium-catalyzed sp2 C-H arylation.1
In the first step of the one-pot, two-step sequence, sulfonylamidine 1 undergoes α sp3 C-H olefination to produce terminal olefins 2E and 2Z (Figure 1). When only this step was performed, good isolated yields were achieved with a variety of electron-donating, electron-withdrawing or sterically-demanding substituents at all R positions. As the barrier to isomerization of the imine bond in these compounds is relatively low, most of the reactions resulted in an approximately 1:1 mixture of the E and Z isomer of the product.
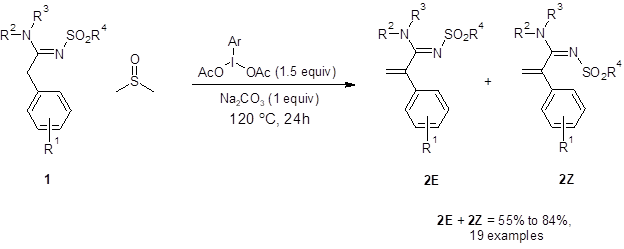
Figure 1: The α sp3 C-H olefination of amidines to form a terminal olefin
When both steps were performed sequentially in the same reaction vessel, good overall yields (51–70%, Figure 2) were achieved in most instances. The addition of aromatic rings with electron-withdrawing substituents at the para position, however, only resulted in trace amounts of the product. Additionally, higher substitution of the amine nitrogen (R2 and R3 = alkyl) also resulted in only a trace amount of the desired product.
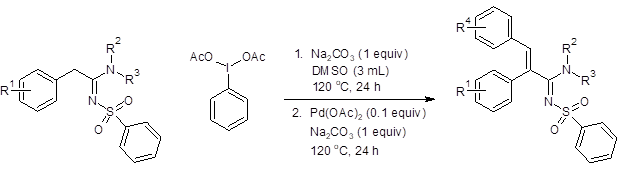
Figure 2: Tandem one-pot synthesis of multisubstitued sulfonylamidines
The mechanism of the olefination reaction was explored using d6-DMSO as the solvent. This resulted in production of the deuterated terminal olefin and indicated that DMSO was the carbon source for the methylene. With this information and with references from the literature, the mechanism below (Scheme 1) was proposed.
In the proposed mechanism, DMSO reacts with the hypervalent iodine reagent which results in the activated DMSO species A. Species A decomposes to the thionium cation B and releases iodosobenzene. Reaction of cation B with the amidine produces the thiomethyl intermediate C which undergoes oxidation to the sulfoxide E through reaction with iodosobenzene. The sulfoxide E then undergoes a Chugaev-type reaction to produce the terminal alkene F. With the addition of the palladium catalyst, alkene F couples with the iodobenzene generated in the olefination reaction to produce the final product G.
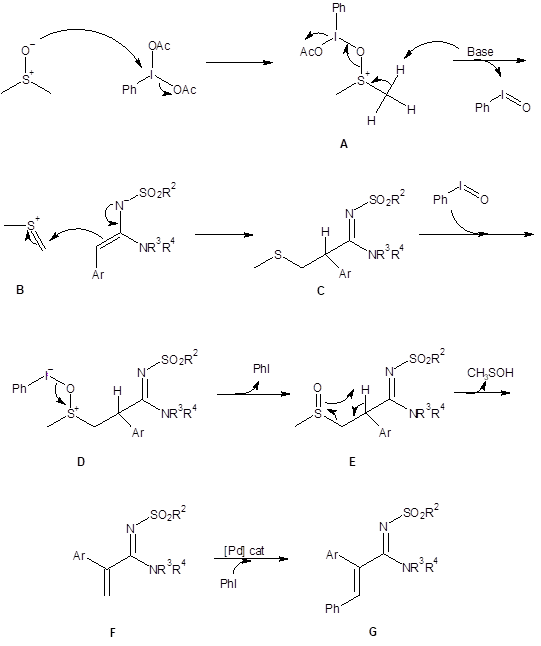
Scheme 1: Proposed mechanism for the olefination reaction with DMSO and phenyliodine diacetate
In summary, this work demonstrates a new metal-free route to produce a terminal alkene at the alpha position of an amidine. DMSO acts as the one-carbon methylene source for this reaction and also functions as the solvent. Additionally, this reaction can be used as the first step of a one-pot terminal olefination and arylation sequence which yields highly substituted sulfonylamidines.
Debra D. Dolliver, Ph.D.
References
1. Pothikumar, R.; Sujatha, C.; Namitharan, K. ACS Catal. 2017, 7, 7783-7787.







Leave a Reply
You must be logged in to post a comment.