CuI/DMSO-mediated synthesis of 3-sulfonyl dihydrofurans
Five-membered rings containing oxygen are common and recurring motifs in biologically-active compounds. Therefore, the development of routes to synthesize these ring systems is of great interest. Recently, Chang et al. developed a one-pot (3 + 2) annulation method to synthesize 3-sulfonyl dihydrofurans (Equation 1).1 This method uses readily-available and relatively inexpensive CuI and DMSO to give excellent yields of functionalized dihydrofurans.
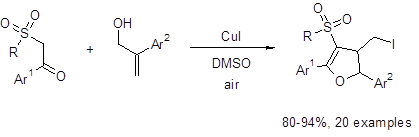
Equation 1: CuI/DMSO-mediated synthesis of 3-sulfonyl dihydrofurans
This route works well with a variety of Ar1 groups, i.e., 4-NO2 and 4-OCH3 substituted phenyl rings. The reaction also tolerated electron-withdrawing and electron-donating groups at the R position, i.e., 4-MeOC6H4 and 4-FC6H4. For Ar2, both a phenyl group and a 4-trifluorophenyl group gave similar yields. However, it was found that an aromatic system was necessary at the Ar2 position as no dihydrofuran was formed when Ar2 was replaced with a hydrogen atom.
These researchers tried several solvents and found that DMSO gave superior yields for this reaction. They concluded that DMSO provided better solubility for CuI than any of the other solvents investigated. Based on previous reports of direct alkylation of active methylenes with the assistance of metal complexation, the authors proposed the following mechanism (Scheme 1).
In this mechanism, Cu2+ is thought to aid in the oxidation of the iodide to iodine. Iodine then assists in the formation of the carbocation species A. Copper is also thought to coordinate to enhance enolate formation. The enolate B then reacts with A to form compound C. Iodine then interacts with the alkene bond in C to produce D which loses a proton and cyclizes to form the product E.
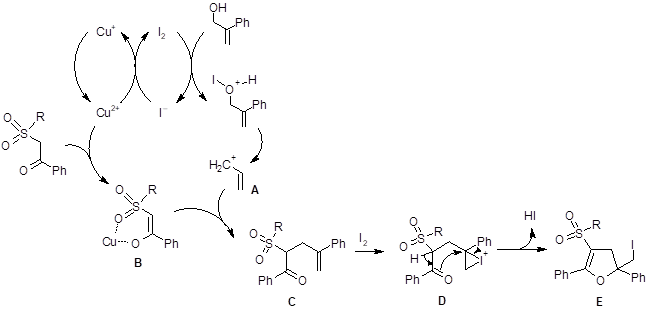
Scheme 1: The proposed mechanism for the formation of the dihydrofuran.
In conclusion, Chang’s group has achieved excellent yields for this (3 + 2) annulation to form interesting sulfonyl dihydrofurans. The reaction requires only readily available CuI and DMSO and can be run under air.
Debra D. Dolliver, Ph.D.
References:
- Hsueh N.-C., Hsiao Y.-T., Chang, M.-Y. Tetrahedron 2017; 73:4398-4406.

Leave a Reply
You must be logged in to post a comment.