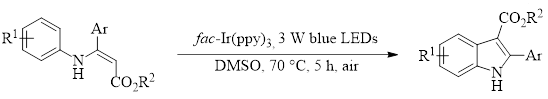DMSO is often the solvent of choice for these types of reactions.2
Wu, et al., have reported a valuable new strategy to synthesize indoles using the photosensitizer fac-Ir(ppy)3 with 3W blue LED lights.3 This reaction starts with the easily-prepared N-arylenamine 1. Indoles exist widely in pharmaceutical, agrichemical, and natural products, so methods to synthesize this heterocycle using green and sustainable means, as reported in the Wu manuscript, are important.
Scheme 1 shows the optimized conditions with a variety of substrates with all giving good to excellent yields of the indole 2. Only one substrate failed to give product (R1 = Et, R2 = NO2, R3 = H), and it was found that this substrate appeared to decompose under the reaction conditions. Interestingly, DMSO was the only solvent that led to a successful reaction. Other polar solvents, such as DMF, MeCN, MeOH, and DCE gave 0% of the product and only led to decomposition of the starting enamine 1. This suggested that DMSO was acting as more than just a solvent and might actually have a role in the reaction.
Scheme 1. Light promoted synthesis of indoles from N-arylenamines
Mechanistic studies indicated that the oxidation/reduction potentials of fac-Ir(ppy)3 and the enamine 1 were affected dramatically by the solvent. Electron spin resonance spectra supported a mechanistic pathway whereby excited fac-Ir(ppy)3* reacts with oxygen to produce a superoxide radical anion (O2·–) in DMSO. The superoxide radical anion then initiates the cyclization of the enamine 1. DMSO is unique in comparison to the other solvents as it allows for the generation of the superoxide radical anion while preventing the formation of singlet oxygen. It is thought that singlet oxygen is generated in the other solvents under these conditions, and it is responsible for the decomposition of the starting material.
In summary, Wu’s team has reported an interesting and high-yielding method to synthesize a variety of functionalized indoles. DMSO is required to generate the superoxide radical anion needed for the reaction. Compared with many traditional methods, this route is relatively simple and mild and works on a variety of N-arylenamines.
Debra D. Dolliver, Ph.D.
References
- (a) Shaw, M. H.; Twilton, J.; MacMillan, D. W. C., Photoredox Catalysis in Organic Chemistry. The Journal of Organic Chemistry 2016, 81 (16), 6898-6926; (b) Romero, N. A.; Nicewicz, D. A., Organic Photoredox Catalysis. Chemical Reviews 2016, 116 (17), 10075-10166.
- He, C.-Y.; Kong, J.; Li, X.; Li, X.; Yao, Q.; Yuan, F.-M., Visible-Light-Induced Direct Difluoroalkylation of Uracils, Pyridinones, and Coumarins. The Journal of Organic Chemistry 2017, 82 (2), 910-917.
- Liu, W.-Q.; Lei, T.; Song, Z.-Q.; Yang, X.-L.; Wu, C.-J.; Jiang, X.; Chen, B.; Tung, C.-H.; Wu, L.-Z., Visible Light Promoted Synthesis of Indoles by Single Photosensitizer under Aerobic Conditions. Organic Letters 2017, 19 (12), 3251-3254.