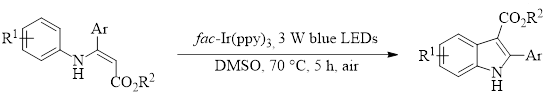Published Account of Synthesizing Indoles Using a Single Photosensitizer and Blue LED Lights
With this in mind, the Wu group has recently published an account of synthesizing indoles using a single photosensitizer [fac-Ir(ppy)3] and blue LED lights to promote a cyclization of easily-prepared enamine substrates in DMSO.1 This reaction produces highly substituted indoles in good to excellent yields (58–99%, 23 examples). Additionally, this reaction requires no special additives or environment and works well when run under air.
This reaction was tolerant of a variety of electron-withdrawing and electron-donating substituents for R1 at all positions on the ring. Likewise, the Ar position tolerated electron-donating and withdrawing substituents in all cases except for para-nitro substitution which afforded no product. The reaction was also unaffected by changes to the ester portion, giving comparable yields with R2 = Et and Me.
A number of control experiments found that in solvents other than DMSO the enamine starting material simply decomposed after irradiation with blue LEDs. Mechanistic studies indicated that the excited photosensitizer reacts with oxygen to produce the superoxide radical anion (O2·–) in DMSO, and this is the species involved in the cyclization reaction. It was also found that DMSO is unique in that it is the only solvent to effectively enhance the oxidation potential of the enamine starting material such that it can undergo the cyclization reaction.
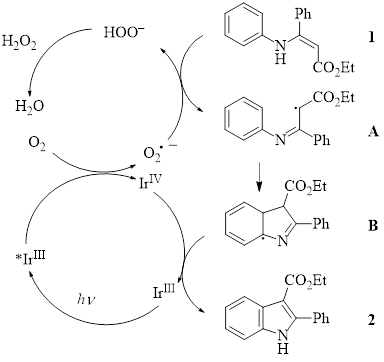
With the experimental evidence for the superoxide radical anion, along with other mechanistic studies (e.g., inclusion of a radical scavenger results in no product formation) the authors proposed the following mechanism.
In this mechanism, IrIII is excited by the blue LED light to *IrIII. Oxygen then interacts with the *IrIII species to form the superoxide radical anion. The superoxide radical anion abstracts a hydrogen atom from the enamine starting material 1 to form the radical intermediate A. This intermediate undergoes radical cyclization for produce B. The cyclic intermediate B is then oxidized by the IrIV species to form the indole product 2.
This straightforward route to indoles is run under air and only requires a single photosensitizer [fac-Ir(ppy)3] and the light from blue LEDs. Therefore, it is extremely mild. DMSO, because of its unique ability to enhance the oxidation potential of the starting material is the only solvent that can be used for this reaction. Compared with traditional indole syntheses, this route is simple to perform and does not require exotic reagents or generate undesirable side products. This looks to be an excellent and green route to produce the indole ring.
Debra D. Dolliver, Ph.D.
References:
1Liu, W.-Q.; Lei, T.; Song, Z.-Q.; Yang, X.-L.; Wu, C.-J.; Jiang, X.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2017, 19, 3251-3254.