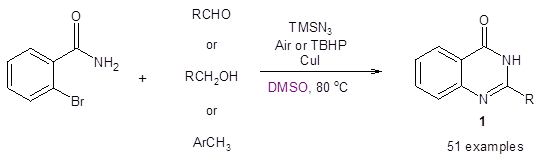An example of a route using a copper catalyst in DMSO has recently been reported by the Tripathi group.
Their paper demonstrates an efficient Cu(I)-catalyzed ligand- and base-free domino strategy to synthesize 2-substituted quinazolinones 1 from either aldehydes, benzyl alcohols, or methyl arenes and 2-bromobenzamide (Equation 1).5 In the optimization of this reaction, it was found that using DMSO as the solvent resulted in significantly higher yields in comparison to other solvents such as DMF, PhCl, PEG-400, dioxane and iPrOH.
The advantages of their quinazolinone synthetic method include the following:
- It doesn’t require expensive or toxic catalysts, ligands, or solvents;
- It has good functional group tolerance; and
- It is an operationally simple domino one-pot reaction which is performed under an air atmosphere.
Equation 1: One-pot Cu(I) catalyzed synthesis of 2-substituted quinazolinones in DMSO
The reaction requires 10 mol% of CuI and 2 equivalents of TMSN3 as the nitrogen source. When R is a substituted phenyl ring, yields are good (generally greater than 70% with only p-nitro substitution giving a lesser yield of 64%). Notably, the reaction tolerates p-bromo substitution on the R aryl group. Heteroaryl R groups are also well tolerated. When R is an aliphatic group or a hydrogen, yields are lower (41-49%).
Mechanistic studies indicate that the copper catalyst and TMSN3 provide anthranilamide (2) in situ. The anthranilamide then undergoes a condensation with an aldehyde [either included as a reactant or generated from oxidation of benzyl alcohol by t-butylhydroperoxide (TBHP) present in the solution]. The imine 3 generated by this condensation then undergoes a cyclization to the dihydroquinazolinone 4 which then is oxidized in air to the quinazolinone 5.
Scheme 1: The proposed mechanism for the reaction to form the quinazolinone starting with either an aldehyde or a benzylalcohol.
When starting with a methyl arene rather than an aldehyde (or when generating the aldehyde in situ by reaction of benzyl alcohol with TBHP), studies indicate that either t-butylphenyl ether and/or t-butylbenzylperoxide is generated in the reaction of the methyl arene with TBHP. These t-butylated species then react with anthanilamide 1 to ultimately form the imine 2 leading to the quinazolinone 5.
The workup for this reaction is simple as most of the quinazolinone products were purified without column chromatography. The paper describes the following purification:
“…ethyl acetate was added to the crude product and the resulting suspension was filtered. Again, the filtrate was concentrated, and this process was repeated 2-3 times to get a good yield of the products.”
This convenient, ligand- and base-free method to form 2-substituted quinazolinones illustrates another use for DMSO in the synthesis of these valuable compounds. The use of DMSO as the solvent in this reaction significantly enhances the yield over other dipolar aprotic solvents and provides for an easy method to isolate pure products.
Debra D. Dolliver, Ph.D.
References
(1) Kim, N. Y.; Cheon, C.-H. Tetrahedron Lett. 2014, 55, 2340.
(2) Mohammed, S.; Vishwakarma, R. A.; Bharate, S. B. J. Org. Chem. 2015, 80, 6915.
(3) Jia, F.-C.; Zhou, Z.-W.; Xu, C.; Wu, Y.-D.; Wu, A.-X. Organic Letters 2016, 18, 2942.
(4) Mahdavi, M.; Hassanzadeh, R.; Soheilizad, M.; Golshani, S.; Moghimi, S.; Firoozpour, L.; Shafiee, A.; Foroumadi, A. Tetrahedron Letters.
(5) Upadhyaya, K.; Thakur, R. K.; Shukla, S. K.; Tripathi, R. P. The Journal of Organic Chemistry 2016, 81, 5046.