Metal-free Aerobic Oxidation to Synthesize Quinazolinones in DMSO
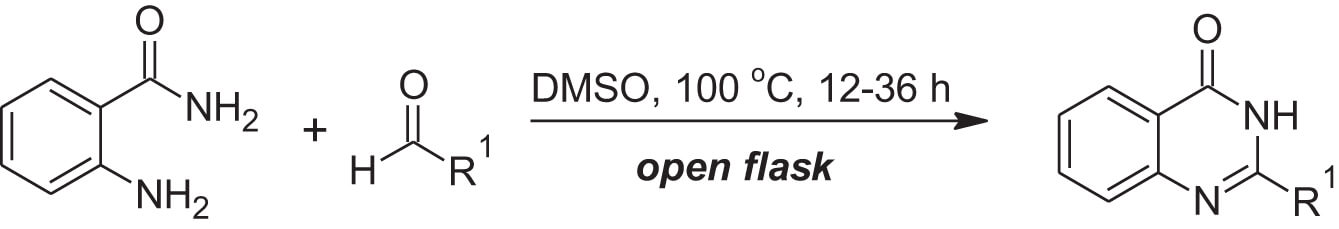
Cheon, et al., have demonstrated a highly environmentally benign route to the synthesis of quinazolinones using readily-available aldehydes and anthranilimide (Equation 1).1 This route proceeds through a metal-free aerobic oxidation in wet DMSO.
Equation 1: Metal-free protocol for the synthesis of quinazolinones in DMSO
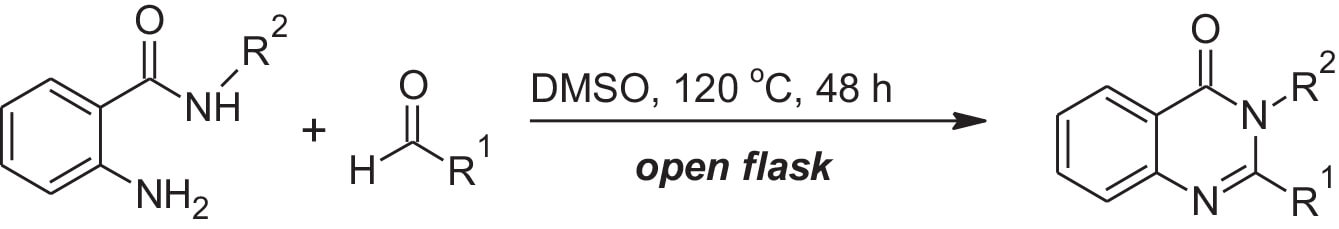
They additionally demonstrated that the protocol also works well with substituents on the amide nitrogen (Equation 2).1
Equation 2: Metal-free protocol with a substituent on the amide nitrogen
In the development of this synthetic route they screened several reaction solvents. As can be seen in Table 1, when performing the reaction with anthranilimide and benzaldehyde, DMSO gave superior yields of the quinazolinone.
Table 1: Comparison yields of the quinazolinone
when starting with anthranilimide and benzaldehyde.
| Solvent | Yield of quanazolinone by1H-NMR (isolated) |
| DMSO | 100% (91%) |
| DMF | 44% |
|
1,4-Dioxane |
12% |
|
H2O |
0% |
|
Toluene |
0% |
|
EtOH |
0% |
In establishing the above protocol in Equation 1, Cheon et al. made the following observations and conclusions:
- The presence of molecular sieves lowered the yield in DMSO.
From this they concluded that water must play a role in the reaction.
- If molecular sieves were present in the reaction in DMSO, the addition of CN– improved the yield.
This indicated a reaction pathway involving a nucleophile.
- No quinazolinone product was formed under an argon atmosphere,
They concluded that oxygen was necessary for the reaction; i.e., the reaction undergoes aerobic oxidation.
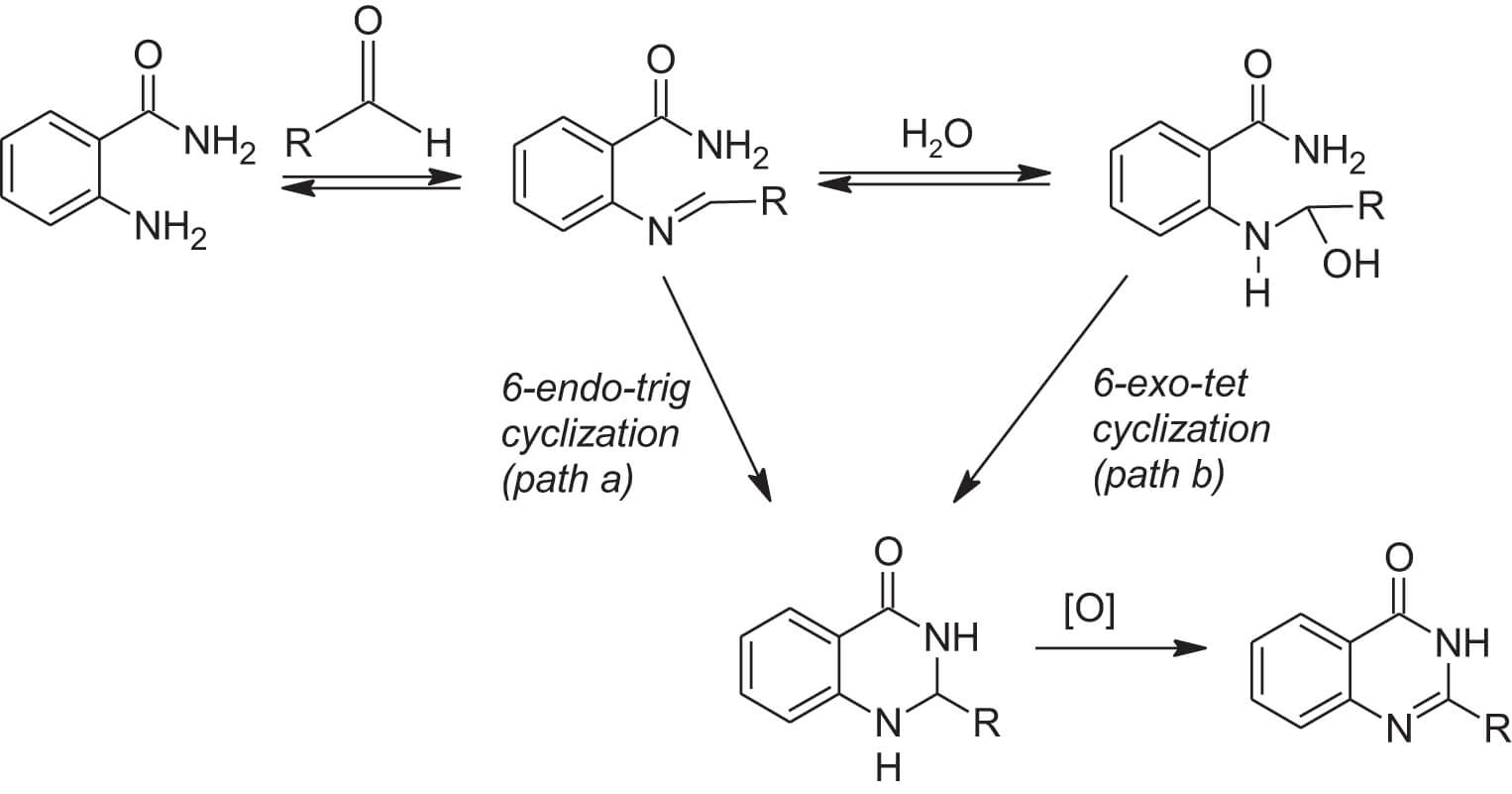
With these observations/conclusions they proposed the following mechanistic pathways (Scheme 1).
Scheme 1: Proposed mechanistic routes for the formation of the quinazolinone.
While both path a and path b are favorable ring closures using Baldwin’s rules,2 the authors indicate that the 6-exo-tet cyclization (path b) must occur at a faster rate than does the 6-endo-trig cyclization (path a). In path b, water (in wet DMSO) acts as a nucleophile to form the carbinolamine species in equilibrium with the imine species. This carbinolamine then cyclizes at a more rapid rate than does the imine species. Hence, a yield enhancement is seen with the presence of a nucleophlic species in the reaction. When molecular sieves are present, water is removed, and only the slower path a is available.
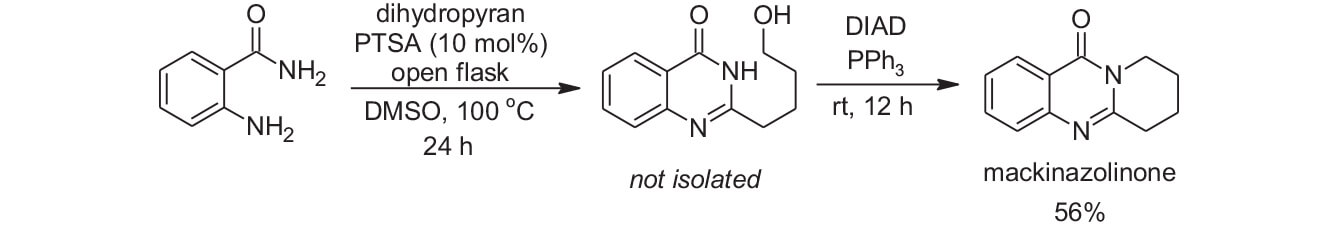
To demonstrate the utility of this reaction, the authors featured it in routes to other useful quinazoline/quinazolinone products. For instance, they report a one-pot synthesis of mackinazolinone (Scheme 2). This is an important natural product tryclyclic quinazoline akaloid known to have a large spectrum of biological activity.3
Scheme 2: One-pot synthesis of mackinazoline.
Dihydropyran/PTSA was used as an aldehyde equivalent in the reaction to form the quinazolinone. The quinazolinone was not isolated and was subjected to a Mitsunobu reaction in the same pot to afford a 56% yield of mackinazolinone. As the authors did not optimize either step in this reaction sequence, they were quite pleased with this result.
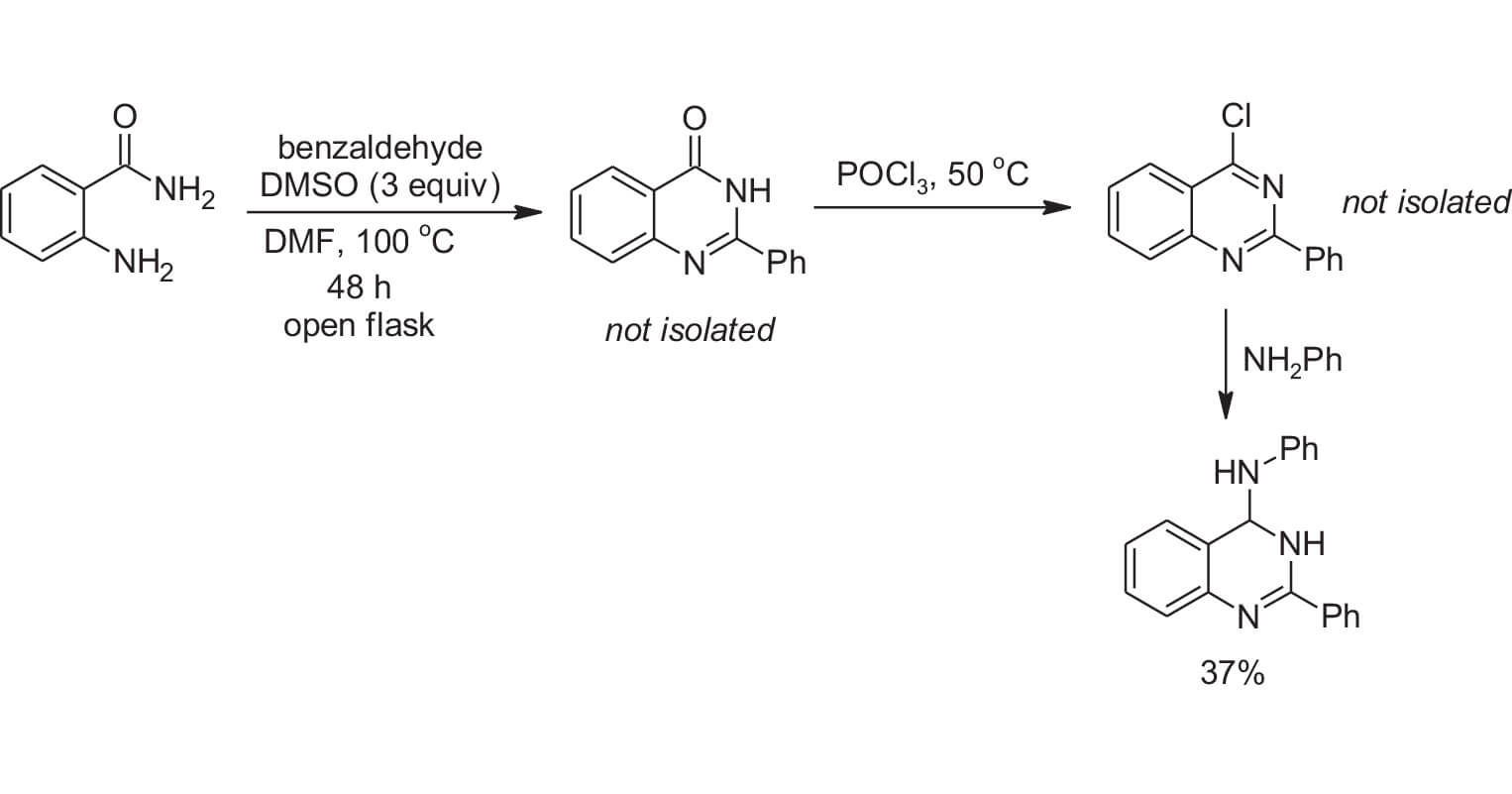
They additionally report a one pot method to further functionalize the quinazoline ring. In Scheme 3 the quinazolinone was formed in a mixture of DMSO/DMF. DMF was added in this first step as DMSO is known to react with POCl3 which is used in the subsequent step. The authors found that even though the amount of DMSO was reduced in the first reaction they still formed the quinazolinone in an adequate amount to proceed. The quinazolinone was not isolated but was reacted with POCl3 to form the 4-chloroquinazoline in situ. Since DMSO was diluted with DMF, there was little of the unwanted reaction between DMSO and POCl3. Subsequently, reaction of the 4-chloroquinazoline with aniline produced the 4-aminoquinazoline in 37% overall yield. Again, the steps in this reaction sequence were not optimized, so the authors were delighted to get a synthetically-useful yield.
Scheme 3: One-pot synthesis of a 4-aminoquinazoline.
In summary, the authors have found an efficient, operationally simple and environmentally benign method to synthesize quinazolinone using anthranilimides, aldehydes and wet DMSO. Additionally, they have effectively applied this method toward the synthesis of biologically/pharmaceutically important compounds.
Debra Dolliver, Ph.D.
References
- Kim, N. Y.; Cheon, C.-H. Tetrahedron Lett. 2014, 55, 2340.
- Baldwin, J. E. Journal of the Chemical Society, Chemical Communications 1976, 734.
- Shakhidoyatov, K. M.; Elmuradov, B. Z. Chem. Nat. Compd. 2014, 50, 781.