Isocoumarins via Pd-Catalyzed Tandem Cascade Reaction
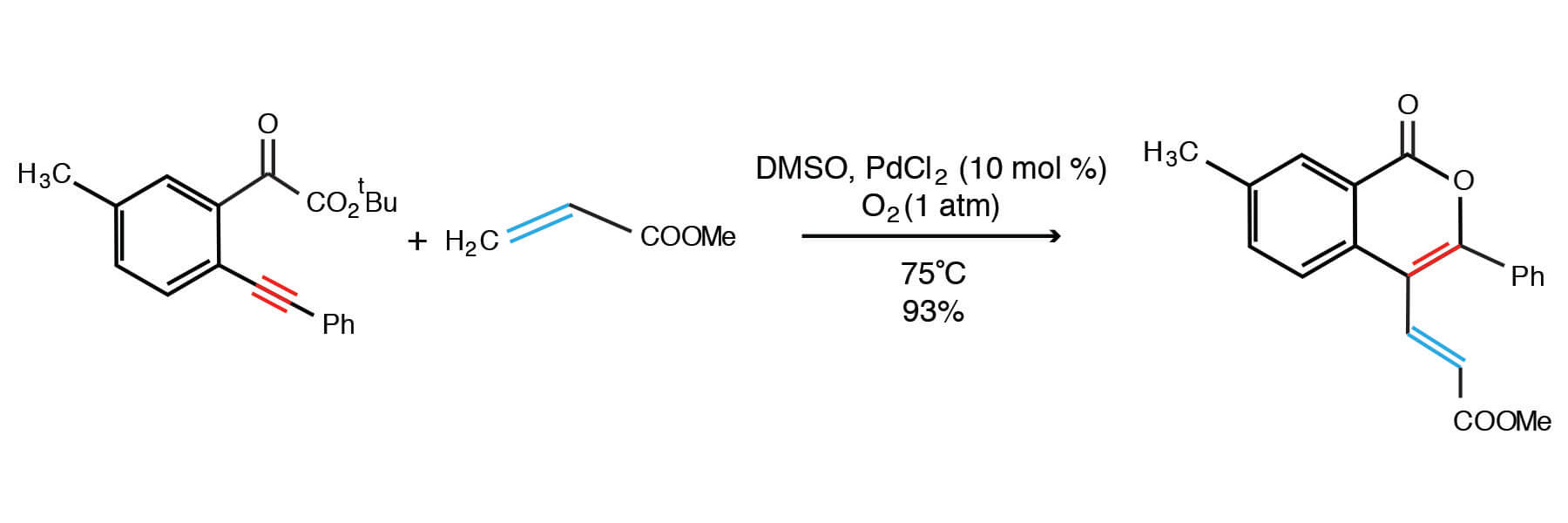
Li, Han, and coworkers of the Dalian Institute of Chemical Physics have reported an isocoumarin synthesis that proceeds through a nucleopalladative-oxidation olefination pathway (1).
Li, Han, and coworkers report the oxidative coupling of olefins and t-butyl 2-alkynylbenzoates to produce isocoumarins (1)
The isocoumarin products formed can possess some degree of complexity, based on the initial functionalization of the starting materials:
- the pendant olefin in the product may be functionalized with aryl or ether groups, based on the choice of olefin ( i.e. methyl acrylate, p-chlorophenyl styrene, etc.)
- The aromatic ring of the arylalkyn-ester substrate seems to tolerate methyl- and chloro-substituents well, and -OMe to a lesser extent.
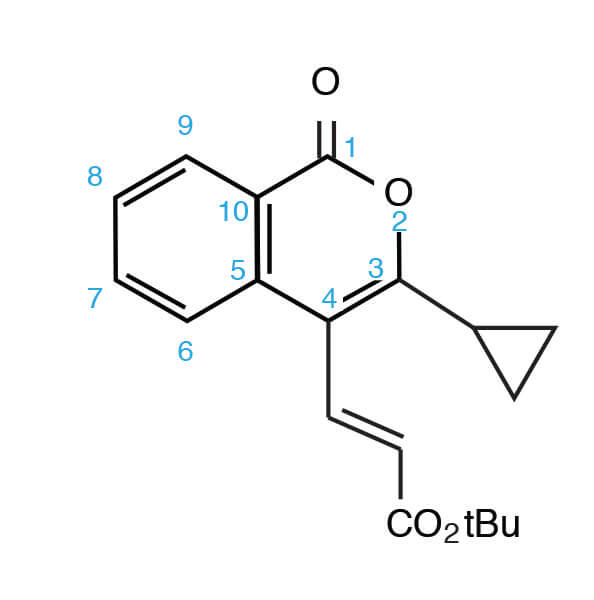
‘numbering’ the atoms in author’s paper: 3- (3-cyclopropyl-1-oxo-1H-isochromen-4-yl)- acrylic tert-butyl ester.
Some background information. This author admits his weak knowledge of this compound class. To appreciate this paper a little review was required. Isocoumarins are somewhat obscure materials, and a first step was a to understand their numbering system.
Once you know the trick, it’s not hard. Carbon ’1′ is the carbonyl carbon, and then you count your way around the ring with the ring oxygen as atom ’2′. One item that was a little puzzling is the use of ’1H’ nomenclature in this compound class. The rule I am familiar with goes this way:
‘In a heterocyclic ring with maximum unsaturation, if the double bonds can be arranged in more than one way, then their positions are specified by nuumbering those nitrogen or carbon atoms which are not multiply-bonded. i.e. bear an ‘extra’ hydrogen atom, by italic capital ‘1H’ ’2H’ ’3H’ etc. The numerals indicate the position of these atoms having the extra hydrogen atom. ’ (2)
You may not have worked with isocoumarins previously; I certainly hadn’t. The authors cited some papers that represented examples in natural products and pharmaceuticals (3). A specific example includes thunberginol A (found in Japanese Hydrangea) (4).
You may be more familiar with coumarin compounds, (the anticoagulant and rodenticide warfarin / coumadin, and ‘coumarin’ itself – a fragrance compound once used to enhance the fragrance of pipe tobacco) (5). The synthesis of these compounds is unrelated to isocoumarin preparation. The final isomeric isocoumarin cousins are the chromones (1,4 benzopyrones).
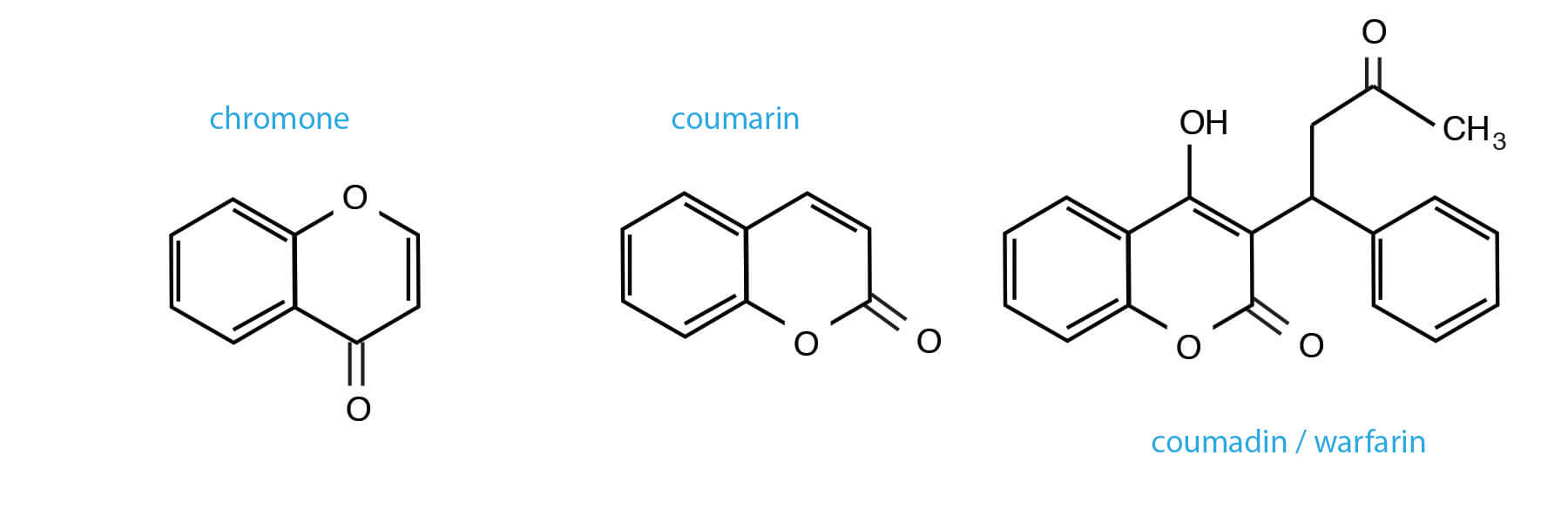
Things that sound like isocoumarin – but which are different.
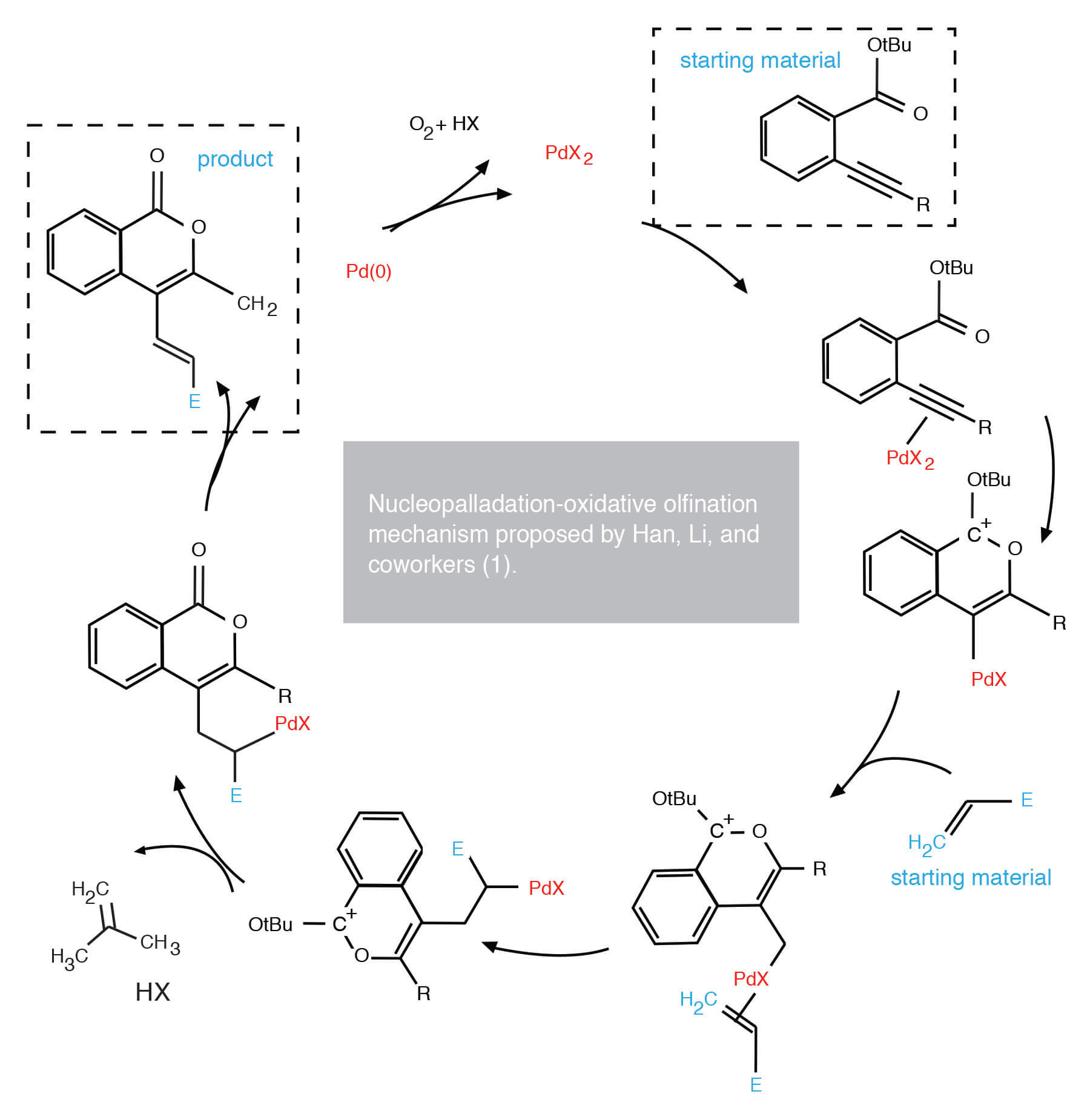
As previously mentioned, the underlying mechanism of their reaction is nucleopalladation, one of three basic ways that Pd-C bonds are formed. Arguable the other two are more familiar to most organic chemists, if only by their associated ‘Name’ reactions:
- Pd-C bond forming method 1: Transmetallation (Stille, Suzuki, Hayama coupling reactions)
- C-H activation reactions via Pd- catalysis.
The authors contend that nucleopalladation provides a benefit in the synthesis of heterocycles in that both C-heteroatom and C-C bonds can be formed in tandem fashion. They spell out the key steps in this process and suggest that palladium plays two important functions in the reaction:
- initial activation of a C-C π bond (that of the 2-alkynyl group) toward intramolecular nucleophilic attack (by the ester carbonyl oxygen)
- final carbopalladation and β-hydride elimination step to produce the olefin group in the 4 position of the molecule.
A capsule summary of the isocoumarin synthesis of Li, Han et Al. follows:
Alkynylester (0.25 mmol), PdCl2 (0.025 mmol), acrylate (0.50 mmol) and DMSO (2.0 mL) were placed in a dry Schlenk tube. The mixture was heated for 16 hours at 85 °C under oxygen (balloon). Workup involved brine washing, DCM extraction (2 x 20 mL), drying over sodium sulfate, and the crude product was purified by silica gel chromatography (pet ether / EtOAc).In closing, a few points about the generality of the reaction:
- The alkynl ester is preferably a t-butyl ester; isopropyl esters seemingly provided lower reaction yields and screening work was performed exclusively using the t-butyl ester compounds.
- 2-alkynlbenzoic acid starting materials failed to provide olefinated products and were not explored.
- DMSO was chosen as reaction solvent due to the recognition by the authors that it is a good solbent in related cyclization-olefination work reported previously by Loh (olefinated isoquinoline synthesis) and Larock (olefinated napthalene synthesis). (6)
Artie McKim
References:
(1) Li, X.; Han, K.; Peng, Z.; Chen, D.; Song;G. J. Org. Chem. 77 (2012), 1579-1584.
(2) Sainsbury, M. Heterocyclic Chemistry (2001), 6.
(3) Subramanian, V.; Rso Batchu, V.; Barange, D.; Pal, M.J. J. Org. Chem. 70 (2005), 4778 b) Mail, R.S.; Babu, K.N. J. Org Chem. 63 (1998), 2488.
(4) Wikipedia.org, “Thunberginol A”. Accessed 26 February 2012.
(5) Wikipedia.org, “Coumarin”. Accessed 26 February 2012.
(6) Larock, R.C.; Doty, M.J.; Han, X.J. J. Org. Chem. 68 (2003) 5936 b) Loh, T.-P.; Feng, C. J. Am. Chem. Soc. 132 (2010), 17710.