DMSO / K2CO3 Terminal Alkyne Synthesis
A recent report by Cheng, Jia, and Kuang (1) of Tongji University is a useful – and pleasantly straightforward- addition to the methods available for alkyne synthesis.
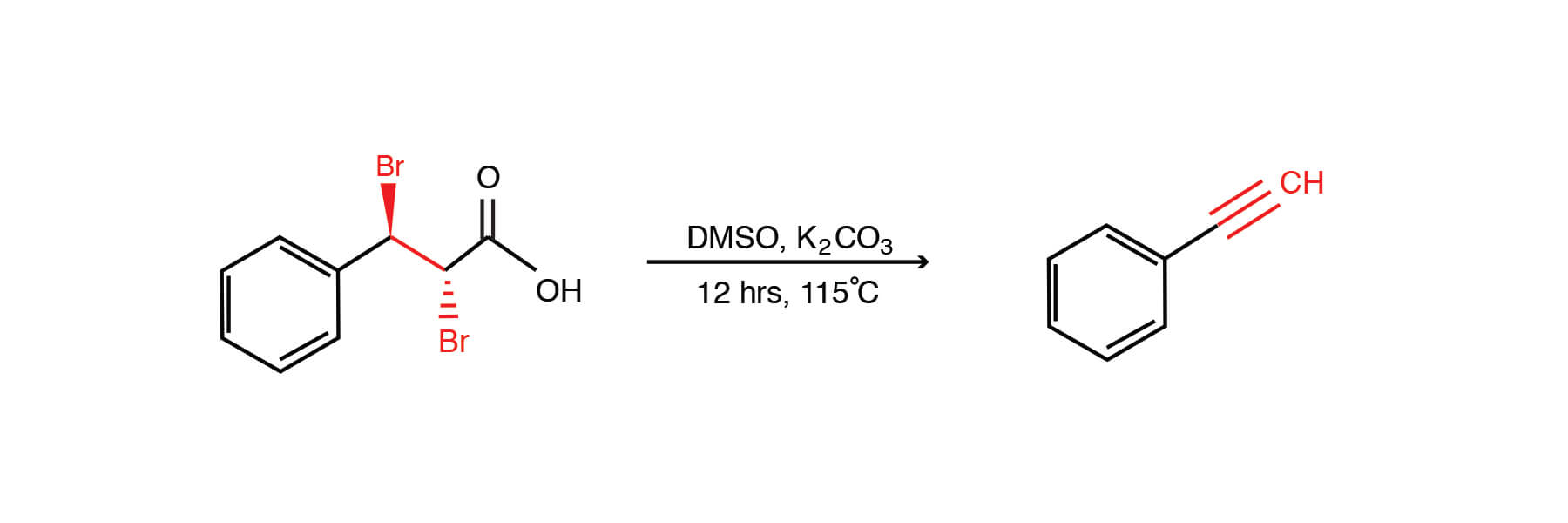
The Terminal Alkyne Synthesis of Kuang et Al. (1)
The alkyne functionality is crucial to modern methods (Sonogashira coupling, ’Click’ chemistry, methathesis reactions) and terminal alkynes can be prepared in a handful of ways. These include the Colvin Rearrangement (addition of lithiated TMS diazomethane to aldehydes), the mechanistically related Gilbert-Seyferth Reaction (dimethyl diazomethyl phosphonate addition to aldehydes), and the Corey-Fuchs olefination (2)with subsequent dehydrohalogenation. In most cases the reagents required for these reactions are relatively exotic. Kuang and coworkers further point out some limitations of these reaction: low temperatures and strong bases are required.
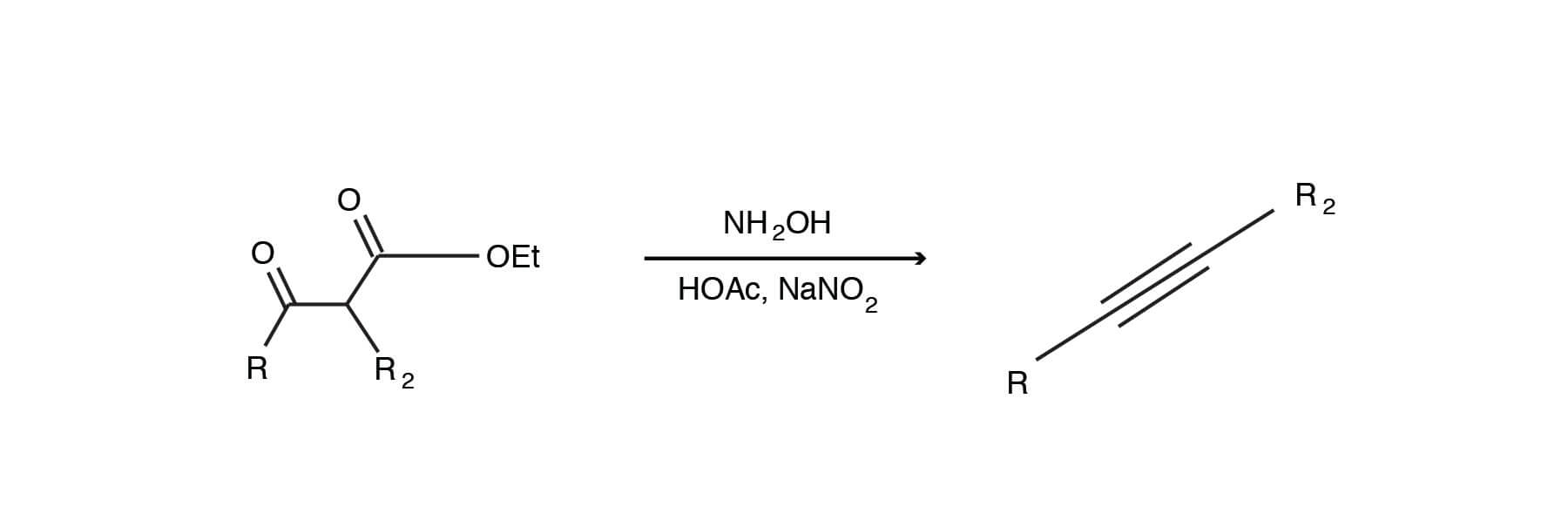
An alkyne synthesis you may not have seen before. Do you know the mechanism? (3)
Their reaction has some nice features:
- Garden-variety cinnamic acids are easily converted to the required 2,3-dibromopropionic acids. These starting materials are inexpensive This is done viabromination in chloroform, under thermal or photochemical means (4).
- Strong base is not required. The E2 trans elimination of HBr is mediated with (mild) potassium carbonate. Base sensitive functional groups are less susceptible than in other alkyne-forming reactions.The authors also suggest that other carbonate salts (Cs2CO3) work, also.
- The overall scope of the reaction is broad (although it was only demonstrated for aryl2,3-dibromoacids). Examples were provided for unfunctionalized and electron-rich ring substituents on the aromatic ring (yields of > 83%); halogenated rings (>90%); and interestingly sterically congested (i.e. ortho-substituted ring) examples (>91%). Good yields were seen for dibromoarene starting materials and pyridine analogues (98 and 72% respectively). The reaction is also tolerant of electron withdrawing ring substituents and examples are provided (p-nitro, p-CF3, p-CO2Me).
A summary of their reaction conditions follows:
Combined were anti-3-aryl-2,3-dibromopropanoic acid (0.5 mmol) , potassium carbonate (1.5 mmol) in DMSO (5 mL). The mixture was stirred at 115°C for 12 hours, when it was cooled and extracted with ether / water (15 / 20 mL, three portions). The organic extracts were combined, dried over sodium sulfate, and the residue obtained post evaporation of the extarction solvent was purified via column chromatography (silica gel / hexanes eluant).Artie McKim.
References
(1) Cheng, X.; Jia, J.; Kuang, C. Chin. J. Chem. 2011, 29, 2350-2354; Org. Process Res. Dev. 2012, 16, 526.
(2) Corey, E. J.; Fuchs, P. L. Tetrahedron Lett. 1972, 13, 3769–3772
(3) See the enjoyable Chem. Commun. 2002, 1555-1563 to read about the ‘Abidi Reaction’. Suggested by Problem 11 of the on-line Harvard practice problem set.
(4) Kim, S.H. ;Wei, H.X.; Willis, S.; Li, G. Synth. Commun. 1999, 29, 4179.