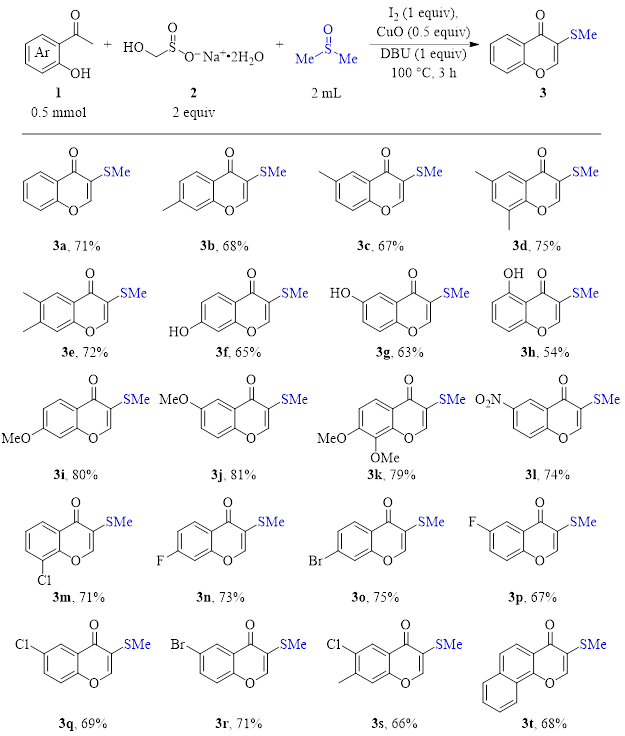
Their method (optimized conditions) is shown in Table 1. Using 2-hydroxyacetophenones 1, Rongalite (2, an inexpensive reagent widely used in industry), and DMSO (in blue), a variety of 3-thiomethyl chromones were synthesized in good yield. This synthesis is one-pot, simple, and requires no metal catalyst or harsh reagents. Therefore, this route is tolerant of a variety of functional groups and substitution patterns.
DMSO/I2-Mediated Synthesis of 3-Thiomethyl Chromones
Chromones are important structural subunits in many natural products and biologically active compounds. Therefore, methods to synthesize or functionalize this structural motif are of high interest. The Wang and Wu group recently published their work where DMSO/I2 facilitates the three-component synthesis of chromones containing a thiomethyl group at the 3-position (3 in Table 1).1 This adds to the growing number of methods whereby heterocyclic annulation reactions are mediated by DMSO/I2.2,3
Table 1: Three-component, DMSO/I2 synthesis of 3-substituted chromones (optimized conditions).
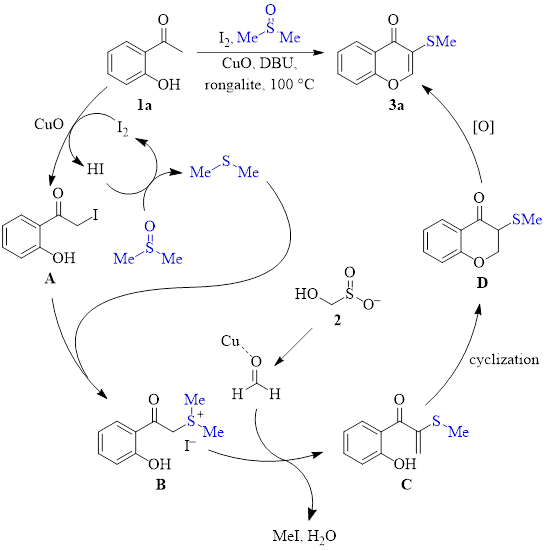
Mechanistic studies led to the mechanism shown in Scheme 1. These studies indicated that formaldehyde and species B are likely formed during the reaction. Additionally, deuterium labeling studies showed that the thiomethyl at the 3-position is derived from DMSO.
This mechanism begins with the 2-hydroxyacetophenone species (1a in this scheme) interacting with molecular iodine to form the α-iodinated species A. Copper(II) oxide may help in this step. DMSO acts to regenerate I2 from HI, resulting in the production of dimethylsulfide. Dimethylsulfide then substitutes I–, producing the intermediate B. Simultaneously, Rongalite (2) decomposes to produce formaldehyde. With assistance from copper coordination, intermediate B adds to formaldehyde, and after loss of water, intermediate C is produced. Cyclization of C to produce chromanone D, followed by oxidation, produces the chromone product 3a.
Scheme 1: Proposed mechanism.
To summarize, the DMSO/I2 pair initiates the reaction of a variety of 2-hydroxyacetophenones with dimethylsulfide (produced from DMSO) and formaldehyde (produced from Rongalite) to yield 3-thiomethylchromones in a one-pot method without the need for exotic or toxic metal catalysts/ligands. Because of the relatively mild conditions of this reaction, the substrate scope is broad, giving good yields with acetophenones having a range of electronic and substitution characteristics.
Debra D. Dolliver, Ph.D.
References:
1Wang, M.; Tang, B.-C.; Ma, J.-T.; Wang, Z.-X.; Xiang, J.-C.; Wu, Y.-D.; Wang, J.-G.; Wu, A.-X. Org. Biomol. Chem. 2019, 17, 1535.
2Gao, F.-F.; Xue, W.-J.; Wang, J.-G.; Wu, A.-X. Tetrahedron, 2014, 70, 4331.
3Org. Lett. 2016, 18, 4388.