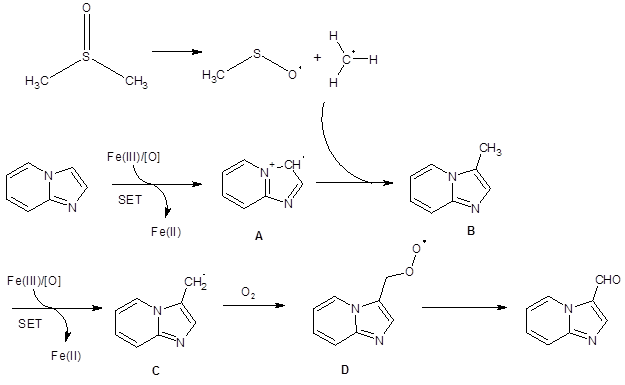A recent paper by Xiang, Chen, and Liu demonstrates how DMSO can efficiently serve as a carbonyl carbon source and as a solvent in the C3-formylation of imidazo[1,2-α]pyridines via iron(III)-catalyzed direct C-H activation under an oxygen atmosphere.1 The optimized conditions are given in Equation 1.
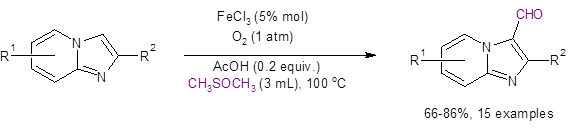
Equation 1: Optimized conditions for the formylation of imidazo[1,2-α]pyridine
The advantages of this method are obvious:
1) All reagents and catalysts have low toxicity;
2) All starting materials are readily available and inexpensive;
3) No ligands are required;
4) The reaction is operationally simple; and
5) The reaction is environmentally friendly.
The reaction was only demonstrated with electron donating substituents (Me, Ph, t-Bu) on the rings, but these substituents were tolerated in all positions. The lowest yield (66%) was encountered when R2 = t-Bu, presumably due to steric crowding at C3.
Mechanistic studies revealed that the yield was low under a nitrogen atmosphere with mostly recovery of unreacted starting material. Radical inhibitors (TEMPO and duroquinone) stopped the reaction. The use of deuterated DMSO in the reaction resulted in completely deuterated formyl groups present in the product.
These studies supported these researchers’ proposed mechanism (Scheme 1).
Scheme 1: Proposed mechanism
In the proposed mechanism, DMSO forms a methyl radical in the presence of ferric salts and oxygen. A single electron transfer reaction to form intermediate A then sets the stage for a radical coupling reaction which forms B. A second single electron transfer oxidation leads to C which traps O2 to form peroxy radical D. Intermediate D then decomposes to form the product.
This mechanism accounts for the experimental observations that DMSO provides the the methine of the formyl group. It also supports the findings that oxygen is necessary for the reaction and that radical inhibitors impede the reaction.
Again, this reaction provides a green, inexpensive and operationally simple technique to regioselectively functionalize imidazo[1,2-α]pyridines. It also provides another example where DMSO acts as a green reagent and solvent.
Debra D. Dolliver, Ph.D.
References
- Xiang, S.; Chen, H.; Liu, Q., Aerobic iron(III)-catalyzed direct formylation of imidazo[1,2-a]pyridine using DMSO as carbon source. Tetrahedron Letters 2016, 57 (34), 3870-3872.