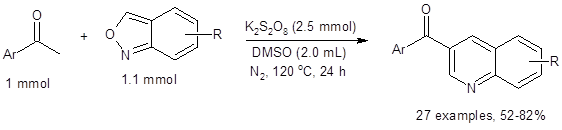The optimized conditions are show in Equation 1. This atom economical and environmentally benign method uses potassium persulfate to facilitate the homologation and annulation reactions. Changing the oxidant and/or solvents dramatically reduces the yield, and in some cases, none of the desired quinoline is produced.
Equation 1: Optimized conditions for synthesis of quinolones
This method is tolerant of substituent variation (electron-withdrawing, electron-donating, protic and bulky substituents along with heteroaryl groups) in the position of the Ar group of acetophenone. The reaction also tolerates a variety of substituents on the anthranil system as seen in Equation 1.
Mechanistic studies support the proposed mechanism given in Scheme 1.
Potassium persulfate interacts with DMSO to produce the electrophile B which reacts with the enolate ion A. Subsequent loss of methylsulfide from C results in the α-β unsaturated species D. Addition of the anthranil to D results in E which cyclizes to produce F. Subsequent dehydration and oxidation produces the quinoline G.
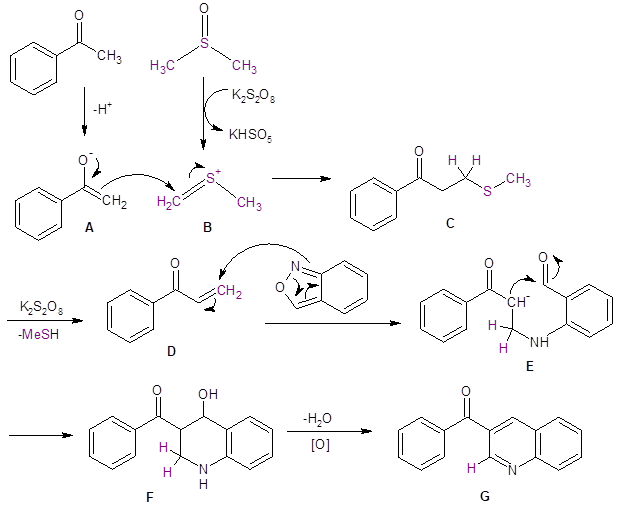
Scheme 1: Proposed mechanism
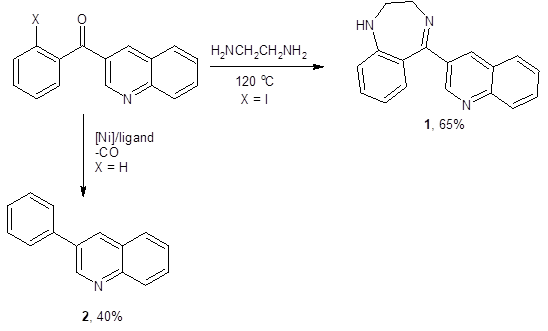
To demonstrate the synthetic utility of this method, this research team developed the one-pot synthesis of pharmaceutically-interesting molecules 1 and 2 in Scheme 2.2 These routes both supplied acceptable to good yields of the target molecules.
Scheme 2: Demonstration of synthetic utility of this method
In summary, this work represents an efficient three-component cascade that provides a relatively green route to 3-ketoquinolines. The reagents and starting materials are readily-available, and there are no toxic or expensive-to-handle waste materials. The yields are generally good, and the reaction has been demonstrated in the one-pot synthesis of biologically active molecules.
Debra D. Dolliver, Ph.D.
References
- Wakade, S. B.; Tiwari, D. K.; Ganesh, P. S. K. P.; Phanindrudu, M.; Likhar, P. R.; Tiwari, D. K., Transition-Metal-Free Quinoline Synthesis from Acetophenones and Anthranils via Sequential One-Carbon Homologation/Conjugate Addition/Annulation Cascade. Organic Letters 2017, 19 (18), 4948-4951.
- (a) Tiwari, D. K.; Phanindrudu, M.; Wakade, S. B.; Nanubolu, J. B.; Tiwari, D. K., [small alpha],[small beta]-Functionalization of saturated ketones with anthranils via Cu-catalyzed sequential dehydrogenation/aza-Michael addition/annulation cascade reactions in one-pot. Chemical Communications 2017, 53 (38), 5302-5305; (b) Morioka, T.; Nishizawa, A.; Furukawa, T.; Tobisu, M.; Chatani, N., Nickel-Mediated Decarbonylation of Simple Unstrained Ketones through the Cleavage of Carbon–Carbon Bonds. Journal of the American Chemical Society 2017, 139 (4), 1416-1419.