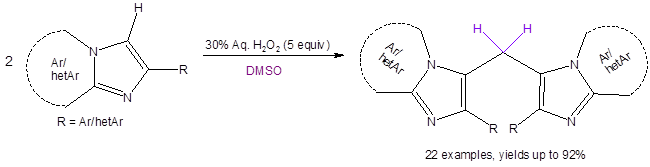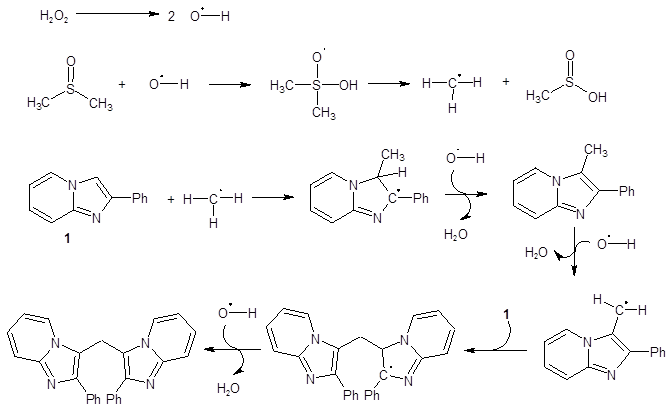
In the effort to readily make such compounds, Yadav, et al., have recently reported the H2O2/DMSO-promoted synthesis of 3,3′-bisimidazopyridinyl methanes (Equation 1).5 This synthetic method is metal-free, regioselective, conducted under air and is amenable to either making symmetrical or unsymmetrical bisimidazo[1,2-a]pyridinyl methanes.
Equation 1: Synthesis of bisimidazo[1,2-a]pyridinyl methanes
In their investigation of substrate scope, it was found that the R group attached to the imidazole ring must be an aryl substituent for the reaction to proceed. It was also found that strongly electron-withdrawing substituents attached on any of the rings diminished the yield. When beginning with a mixture of two different imidazole starting materials, a mixture of products was encountered. However, if the electronic demands (electron-withdrawing vs. electron-donating) of the two imidazoles were different, the unsymmetrical bisimidazo[1,2-a]pyridinyl methane product was dominant in the product mixture.
Mechanistic studies revealed that the bridging methylene group does arise from one of the methyl groups of DMSO. This was substantiated by the finding that when deuterated DMSO was used in the reaction, the resulting bridging methylene group was also deuterated. Further mechanistic explorations pointed to the reaction proceeding through a radical pathway as the addition of radical inhibitors like TEMPO or BHT resulted in no product formation. These findings suggested the mechanism shown in Scheme 1.
Scheme 1: Proposed mechanism
The proposed mechanism helps to explain the observed regiochemistry and the necessity of having an aryl group substituent on the imidazole ring.
This reaction provides a facile, green, and metal-free route to these 3,3′-bisimidazopyridinylmethanes. DMSO provides the methylene group to link two imidazoles regioselectively. In general the yields are good. This work adds to the growing number of reactions where DMSO plays the role of a methylene group synthon.
Debra D. Dolliver, Ph.D.
References
(1) Jones-Mensah, E.; Karki, M.; Magolan, J. Synthesis 2016, 48, 1421.
(2) Wu, X.-F.; Natte, K. Advanced Synthesis & Catalysis 2016, 358, 336.
(3) Liu, P.; Shen, Z.; Yuan, Y.; Sun, P. Organic & Biomolecular Chemistry 2016, 14, 6523.
(4) Li, P.; Weng, Y.; Xu, X.; Cui, X. The Journal of Organic Chemistry 2016, 81, 3994.
(5) Patel, O. P. S.; Anand, D.; Maurya, R. K.; Yadav, P. P. The Journal of Organic Chemistry 2016.
(6) Parekh, K. D.; Dash, R. P.; Pandya, A. N.; Vasu, K. K.; Nivsarkar, M. Journal of Pharmacy and Pharmacology 2013, 65, 1785.