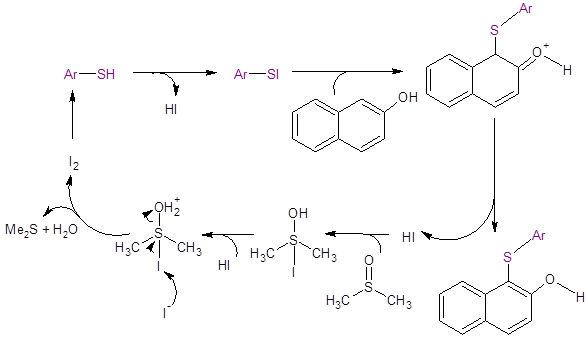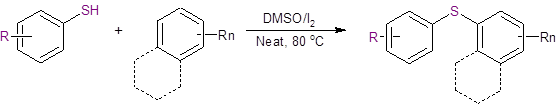
Equation 1: DMSO/I2 catalyzed C-H activation and C-S bond formation reaction
This reaction displays the following benefits over other reported methods to synthesize aryl sulfides:
- There is no need for prefunctionalization of either coupling partner.
- The reaction works best without solvent.
- The catalyst system is inexpensive.
- The reaction is metal-free, so there is no concern about metal contamination of the product.
- There are no air-sensitive reagents.
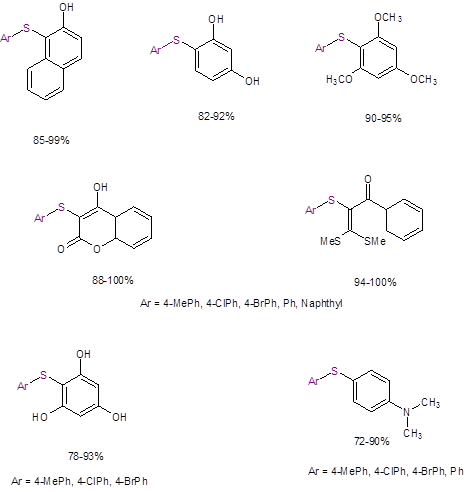
The isolated yields for all cases were excellent (Figure 1). The reaction tolerated electron donating and electron-withdrawing substituents on the thiol. It was demonstrated with only electron donating substituents on the other coupling partner. The reaction also worked well with steric crowding in this other coupling partner.
Figure 1: Isolated Yields
Based on literature reports of the formation of ArSI from disulfides and I2,2,4,5,7 the authors propose a mechanism for their work involving this species. They speculate that ArSI is formed in situ when the thiol and I2 are present. The ArSI then liberates I– and the electrophile ArS+ which reacts via an electrophilic aromatic addition pathway with an aromatic ring containing an electron donating group. The I2 catalyst is regenerated from I– through a redox reaction with DMSO.
Figure 2: Proposed Mechanism
The experimental procedure follows a straightforward and simple method in which the reactants and catalysis system are simply heated on an oil bath at 80 oC for 3 hours. Iodine catalyst is present at 10% load; DMSO is present at 2 equivalents. The reaction is conducted under ambient atmosphere, and the workup involves a simple extraction and purification by column chromatography.
Debra D. Dolliver, Ph.D.
References
(1) Venkateswarlu, V.; Aravinda, K. K. A.; Gupta, S.; Singh, D.; Vishwakarma, R. A.; Sawant, S. D. Org Biomol Chem 2015, 13, 7973.
(2) Azeredo, J. B.; Godoi, M.; Martins, G. M.; Silveira, C. C.; Braga, A. L. J. Org. Chem. 2014, 79, 4125.
(3) Jagadhani, S. G.; Kundalikar, S. G.; Karale, B. K. Orient. J. Chem. 2014, 30, 2073.
(4) Ge, W.; Wei, Y. Green Chem. 2012, 14, 2066.
(5) Ge, W.; Zhu, X.; Wei, Y. Adv. Synth. Catal. 2013, 355, 3014.
(6) Parumala, S. K. R.; Peddinti, R. K. Green Chem. 2015, 17, 4068.
(7) Liao, Y.; Jiang, P.; Chen, S.; Qi, H.; Deng, G.-J. Green Chem. 2013, 15, 3302.