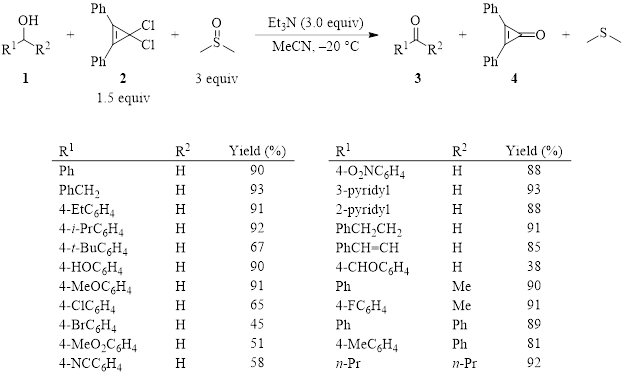
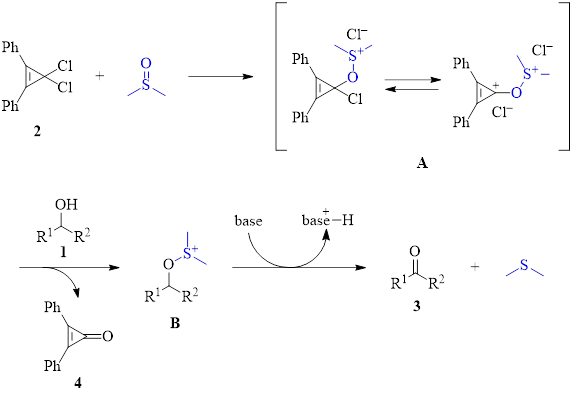
Oxidations of primary alcohols using activated DMSO to produce aldehydes are important reactions. Oxalyl chloride is a common activator for DMSO (Swern oxidation) and has been widely used. However, oxalyl chloride has some drawbacks in that it is volatile and toxic. Additionally, the oxalyl chloride/DMSO pair must be kept cold (less than –60 °C) to avoid a highly exothermic and uncontrollable reaction. Recently, a new method using a 3,3-dichlorocyclopropene (2) as the activator of DMSO has been found to result in the selective oxidation of primary alcohols (1, R2 = H) to aldehydes at a much higher temperature of –20 °C.1
As can be seen in Table 1, the optimized conditions for the dichlorocyclopropene activation of DMSO generate the product in good to excellent yields for a variety of starting materials with varying electronic demands and steric constraints. It should be noted that the dichlorocyclopropene activator 2 must be freshly synthesized by reacting 2,3-diphenylcyclopropenone (4) with oxalyl chloride. In other words, like the traditional Swern oxidation, oxalyl chloride cannot be avoided, making the chief advantage of this new method the fact that it can be executed at higher temperatures. The activator 2 can be regenerated from recovered byproduct 4 by subsequent reaction with oxalyl chloride.