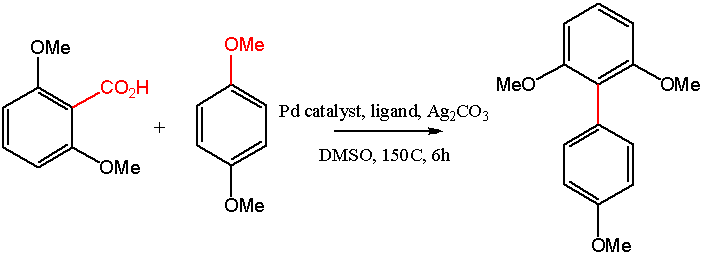
Diarylalkynes Coupling in DMSO
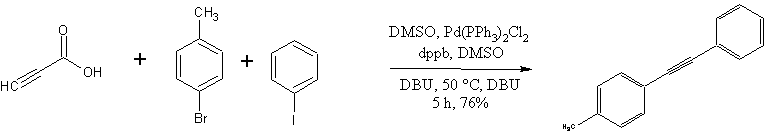
The diarylalkyne synthesis of Park, Song and coworkers (1).
Conjugated alkynes have potential in nanoelectric materials and conductive organic films. The authors describe the disadvantages of some of the established ways to make this compound class:
- The traditional Sonagashira reaction: requires a copper cocatalyst
- The use of acetylene as starting material: difficult to handle at the preparative scale.
‘Protected’ acetylenes are easier to work with, but also have issues:
- Stille-type coupling of distannylalkynes: stoiciometric amounts of hazardous tin waste are generated
- bis-(trimethylsilyl)acetylenes require an excess of strong base.
The authors have developed the use of propiolic acid (propynoic acid) as the alkyne.
Another decarboxylative coupling: Becht et Al. (2)
component in the palladium-catalyzed coupling of various aryl halides. Decarboxylativecoupling reactions are a relatively newdevelopment, with the advantage that innocuous CO2 is a byproduct. The Becht group (2) is active in this area and has published a decarboxylative coupling route to sterically hindered biaryl compounds.
Previous work in this area by the authors had produced a decarboxylative coupling reaction based on aryl- or alkyl alkynyl carboxylic acids (3). Advances toward an ecofriendly method were made, but they cite some limitations of this earlier work, including:
- excess of TBAF required (expensive, and TBAF is moisture sensitive)
- relatively high Pd loading (costly)
- ligand stability (P-tBu3) problematic.
Screening experiments using a model system (bromobenzene + propiolic acid) led them to these conditions:
- Pd source: Pd(PPh3)Cl2 was chosen; other sources evaluated were Pd(PPh3)2(OAc)2, Pd2(dba)3, Pd(OAc)2 and Pd(PPh3)4
- Base: DBU gave a quantitative yield with Pd(PPh3)Cl2. Twelve other bases were tried, including the somewhat exotic 1,8-diaminonapthalene.
- Solvent: DMSO produced the highest product yields of solvents tested. Other solvents evaluated included toluene, diglyme, xylene, and NMP. Interetingly, although NMP has solvent properties which are similar to those of DMSO, its use only resulted in a 23% yield.
- Ligand: dppb outperformed PPh3, P t-Bu3, PCy3, dppf, and Xantphos.
Ultimately, their general procedure might paraphrased in this way:
Pd(PPh3)2Cl2 (105 mg, 0.15 mmol), dppb (128 mg, 0.30 mmol), aryl halides (6 mmol) and propiolic acid (212 mg, 3 mmol) were mixed with DBU (913 mg, 6 mmol) in a small RBF. DMSO (15 mL) was added and the the sealed flask was heated at 80C for 3 hr.
The reaction mixture was poured onto saturated NH4Cl (aq) and extracted with ether. Ether extracts were washed with brine, dried over magnesium sulfate, filtered, and concentrated in vacuo to provide a residue for purification by flash chromatography (ethyl acetate / hexane).
Lee, Song and coworkers also described the use of 2-butyndioic acid as a substitute for propiolic acid, and yields of symmetrical diarylalkynes are in most cases excellent.
Yields of unsymmetrial diarylalkynes from propiolic acid suffer somewhat, but yields of 65-89% were reported. An interesting feature of this reaction is that it apparently works best when a) one aryl halide is a bromoarene and the other an iodoarene and b) both arenes are added in the beginning of the reaction.
The authors also describe mono- vs. di-coupling via temperature control, and state that this work represents the first successful report of double decarboxylative coupling using 2-butyndioic acid.
Artie McKim.
(1) Park, K.; Bae, G.; Moon, J.;Choe, J.; Song, K.H.; Lee, S. J. Org. Chem. 2010, 75, 6244-6251.
(2) Becht, J.-M.; Catala, C.; Drian Claude, L.; Wagner, A. Org Lett 2007, 9, 1781-3.
(3) Moon, J.; Jang, M.; Lee, S. J. Org. Chem. 2009, 74, 1404-1406 b) Kim, H. ; Lee, P.H. Adv. Synth. Catal. 2009, 351, 2827-2832.