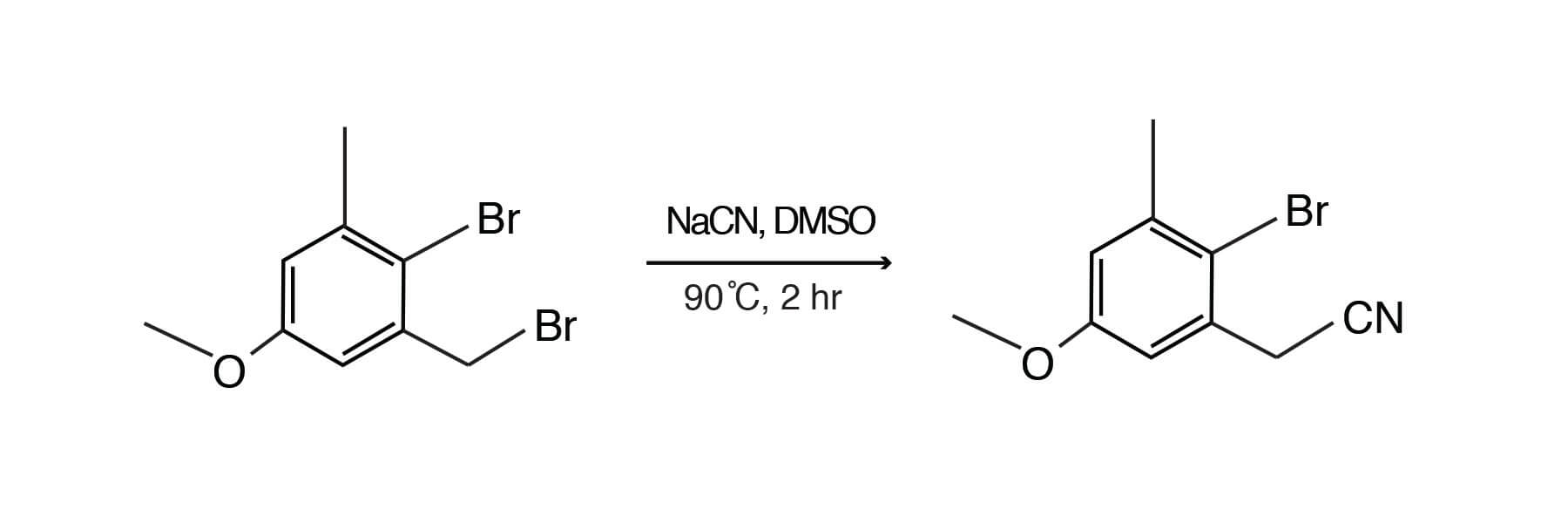
Perhaps the most widely used of the displacement reactions in DMSO are those involving the cyanide ion. Halogen atoms and sulfonate (tosyl) groups are displaced rapidly by cyanide ion. The yields of the desired products are often higher and side reactions are minimized in DMSO. Shown is nucleophilic displacement of a benzylic bromide using sodium cyanide to produce (2-bromo-5-methoxy-3-methylphenyl)-acetonitrile. [Rucker, Mark; Brueckner, Reinhard; Synlett, 1997; 1187-1189]
Cyanation and DMSO
Many inorganic cyanides are more soluble in DMSO than in other organic solvents. DMSO can be used advantageously in systems where water is undesirable. At 95°C, about 10 g of sodium cyanide and/or 2 g of potassium cyanide will dissolve in 100 cc of DMSO. At 25°C, 1 g of either is soluble [Cisney, M. E. Crown Zellerbach, unpublished results (Mar. 20, 1967) and (Feb. 13, 1967)]. Bulletin 105B contains additional information on solubility at various temperatures.
DMSO also dissolves mercury cyanide, cadmium cyanide, and mixtures of potassium cyanide with copper, nickel, zinc, cobalt, or silver cyanides. These mixtures appear to be complex salts [Dodd, R.E., Gasser, R.P.H., Proceed. Chem. Soc. 415] In many cases it is not necessary to have complete solubility of sodium cyanide in DMSO. Reactions can be run using an agitated, stirred slurry of sodium cyanide with DMSO. Yields are commonly good with primary aliphatic halides, but are somewhat lower with secondary halides due to dehydrohalogenation [Friedman, L., Shechter, H. , J. Org. Chem. 25, 877-879 (1960); Smiley, R.A., Arnold, C. , J. Org. Chem.25, 257-258 (1960); Guenther, H. J., Jaeger, V., Skell, P. S., Tetrahedron Lett. 2539-2542 (1977) ].

