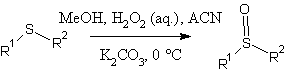
The sulfide oxidation procedure of Page and coworkers (1)
Our first trial was based on the work of Page and coworkers at the University of Liverpool (1).
A first look at this procedure suggested it would be simple to implement in the lab and could be used to produce preparative quantities of the sulfoxide we required. Features that made this reaction attractive include:
- relatively brief reaction times (under 2 hours)
- good to excellent product yields (69-91%)
- No exotic reagents are required.
The mechanism of peroxide activation involves a peroxyimidic acid formed in situ by
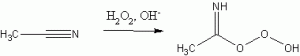
Peroxyimidic acid intermediate formation.
Reaction between acetonitrile and hydrogen peroxide. The authors mention the need to minimize the concentration of this intermediate as it can decompose to acetamide if allowed to react again with peroxide (2). This is achieved by using a large excess of acetonitrile and through the slow introduction of peroxide. As previously mentioned, this is essentially a modification of the Payne epoxidation of olefins; Page and coworkers have extended this to sulfoxide synthesis.
Based on their experimental procedure and some preliminary tests, this is the procedure we put together for our purposes:
Prepare Mixture 1: dialkylsulfide (0.013 mol, MR 1) and acetonitrile (0.84 g, 0.020 mol, MR 1.53) were dissolved in reagent MeOH (50 mL). K2CO3 (13.21g, 1.380 mol, MR 1.49) and magnetic stir bar were added; the mixture was cooled to O°C. Prepare Mixture 2: H2O2 (2.06 g of fresh 50% solution; 1.03g active basis, 0.015 mol, 1.13 MR) was dissolved in MeOH (25 mL) and cooled to O°C. Mixture 2 was added slowly by syringe over 30 minutes. The resulting mixture was stirred at O°C for three hours and allowed to warm to room temperature over night. Saturated Brine (20mL) was added and this was extracted with dichloromethane (4 x 25 mL).The progress of the reaction was followed by FID-GC. When the mixture was worked up we found the following:
- all of the sulfide starting material had been consumed.
- Although the reaction is selective to sulfoxide formation, a considerable amount of sulfone byproduct was formed also.
In our hands, a contained yield of about 70% was the best we could do. This was after several small-scale trials wherein we varied the following:
- reaction time: we saw that little reaction had occurred after two hours, and prolonged the reaction time realtive to the generic procedure given in the paper.
- peroxide addition time and dilution: clearly overoxidation can be minimized by controlling this variable, and we worked to add the peroxide as slowly as possible. The Page paper does not prescribe a dilution volume for the aqueous peroxide (‘added dropwise as a dilute methanol solution’) and this could be a worthwhile factor to optimize.
Our conclusion:
this is a serviceable reaction which is easy to perform. We suspected that a more selective method was available, however, and did not scale this reaction to produce a larger batch for purification. If we wanted to improve the selectivity of the reaction, some things we would look into would include:
- The authors stated that a large excess of acetonitrile can minimize overoxidation. The generic experimental procedure in the Page paper recommended a slight excess (1:1.5 molar ratio relative to starting sulfide). Would a larger amount of acetonitrile make a difference?
- It appears that the paper we followed used a substoichiometric amount of potassium carbonate. We thought a larger amount of base might make a difference but didn’t examine this.
- Further consideration of temperature / time conditions might improve product yields.
Artie McKim
References
(1) Page, P.C.B.; Graham, A.E.; Bethell, D.; Park, B.K. Synthetic Communications 23, 11 (1993) 1507-1514.
(2) The Radziszewski Reaction was a new one to me. It is presumably a useful way to convert nitriles to amides, when other functional groups present are resistant to oxidation conditions. Page and coworkers provide this reference: Ogata, Y. ; Sawaki, Y. Tetrahedron 20 (1964), 2065.

