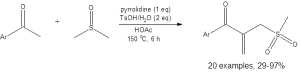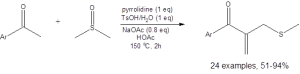
Equation 1: The optimized conditions for the coupling of DMSO with a methyl ketone to access a ketoallylmethylsufide
This is not the first example of DMSO being used as an “ideal sulfur source” due to its cost efficiency. For instance, DMSO has served as the electrophile in Pummerer rearrangement reactions,3 and it has served as the methylthio or the methylsulfinyl group in various metal-catalyzed transitions.4 Despite advances, these reactions have generally been limited to reactions that only involve arenes, alkenes, or alkynes as nucleophiles. The work reported in the Wen paper is the first example of direct coupling of DMSO with the C(sp3) carbon of methyl ketones. It is also novel in that it only requires acid promotion without the need for a metal catalyst.
Equation 2: The optimized conditions for the coupling of DMSO with a methyl ketone to access a ketoallylmethylsufone
The reactions reported in Equations 1 and 2 are generally high yielding and tolerate a variety of functional groups. The reaction in Equation 1 tolerates electron-donating and electron-withdrawing substituents and ortho, meta, and para placement of substituents on the aromatic ring. This reaction also is amenable to heteroaryl groups. Only six of the 24 reported yields were less than 70%.
The formation of the sulfone, illustrated in Equation 2, is also generally high yielding, with only six of the 20 reported examples having yields below 70%. (Only two had yields below 50%.)
Mechanistic studies (including deuterium-labeled DMSO and substrate studies, reactions beginning with proposed intermediate species, reactions with phenylmethylsufoxide, and studies using radical inhibitors) all supported the proposed mechanistic pathway to the sulfide (Scheme 1) and, minus the NaOAc, the proposed mechanistic pathway to the sulfone (Scheme 2).
In Scheme 1, DMSO is activated by the acid to produce the electrophilic thionium ion A. Reaction with enamine B produces the thiomethylated enamine C. Attack of C by another thionium ion makes the dithiomethylated species D. Reaction with water produces the dithiomethylated ketone E which undergoes elimination in the presence of base to make the ketoallylmethylsufide F.
Scheme 1: Proposed mechanistic pathway for the formation of the ketoallylmethylsufide.
Mechanistic studies indicated that the sulfone species K was formed through a radical pathway (Scheme 2). It had been previously reported5 that DMSO in acetic acid in the presence of TsOH generates species G which cleaves to form the methylsulfonyl and the thiomethyl radicals H and I. Radical H then adds to the ketoallylmethylsufide F to produce J. Loss of a thiomethyl radical produces the sulfone K, and the thiomethyl radical gets oxidized to the sulfone radical to reenter the reaction pathway.

Scheme 2: Proposed mechanistic pathway for the formation of the ketoallylmethylsufone.
The reactions developed by Wen’s group offer an alternative, economically-attractive route to valuable sulfur-containing organic compounds, and is another tool for the synthetic chemist’s toolbox.
Debra D. Dolliver, Ph. D.
References
- (a) Gómez, J. E.; Guo, W.; Kleij, A. W., Palladium-Catalyzed Stereoselective Formation of Substituted Allylic Thioethers and Sulfones. Organic Letters 2016, 18 (23), 6042-6045; (b) Gao, W.; Lv, H.; Zhang, X., Rh/DuanPhos-Catalyzed Asymmetric Hydrogenation of β-Acetylamino Vinylsulfides: An Approach to Chiral β-Acetylamino Sulfides. Organic Letters 2017, 19 (11), 2877-2880.
- Wen, Z.-K.; Liu, X.-H.; Liu, Y.-F.; Chao, J.-B., Acid Promoted Direct Cross-Coupling of Methyl Ketones with Dimethyl Sulfoxide: Access to Ketoallyl Methylsulfides and -sulfones. Organic Letters 2017, 19 (21), 5798-5801.
- Smith, L. H. S.; Coote, S. C.; Sneddon, H. F.; Procter, D. J., Beyond the Pummerer Reaction: Recent Developments in Thionium Ion Chemistry. Angewandte Chemie International Edition 2010, 49 (34), 5832-5844.
- (a) Sharma, P.; Rohilla, S.; Jain, N., Copper Acetate-DMSO Promoted Methylthiolation of Arenes and Heteroarenes. J. Org. Chem. 2015, 80 (8), 4116-4122; (b) Pramanik, M. M. D.; Rastogi, N., Visible light catalyzed methylsulfoxidation of (het)aryl diazonium salts using DMSO. Chemical Communications 2016, 52 (55), 8557-8560.
- Freeman, F.; Angeletakis, C. N., Formation of .alpha.-disulfoxides, sulfinic anhydrides, and sulfines during the m-chloroperoxybenzoic acid oxidation of symmetrical S-alkyl alkanethiosulfinates. Journal of the American Chemical Society 1983, 105 (12), 4039-4049.