*Exceptions: when R2 = 4-Me and R1= 4-CF3, 49%; 4,5-dimethyl 10%; 4-NO2, 0%. One example of aliphatic aldehyde also gives a good yield.
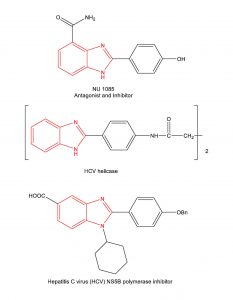
The synthesis of benzimidazoles has received considerable attention as this scaffold is found in several commercial drugs (Nexium, Attacand, Protonix, Prilosec, Famvir) and in several experimental drug candidates.
Despite the interest in benzimidazoles, many of the main synthetic strategies either require harsh conditions, highly functionalized starting materials, and/or expensive catalysts.2-6 These issues make the Punniyamurthy route particularly attractive as it uses readily-available and inexpensive starting materials and the base metal copper as a catalyst. Copper is abundant, cheap, and nontoxic, and there are other recent examples of it acting as a catalyst in the synthesis of valuable nitrogen heterocycles in DMSO.7,8 In fact, there has been another report recently of the synthesis of benzimidazoles using a copper catalyst in DMSO.5 (See “One Pot Benzimidazole Synthesis” in a previous post from February 5, 2012.)
It is noteworthy that DMSO out-performed all other solvents in the Punniyamurthy synthesis. For example, in the model reaction used for optimization the isolated yields dropped significantly with other solvents (Table 1).
Example Experimental Drug Candidates containing the benzimidazole scaffold
Table 1: Isolated Yields during Optimization
| Solvent | Isolated Yield % |
| DMSO | 72 |
| DMF | 16 |
|
Toluene |
5 |
|
ACN |
60 |
|
THF |
3 |
|
DCM |
3 |
Despite the interest in benzimidazoles, many of the main synthetic strategies either require harsh conditions, highly functionalized starting materials, and/or expensive catalysts.2-6 These issues make the Punniyamurthy route particularly attractive as it uses readily-available and inexpensive starting materials and the base metal copper as a catalyst. Copper is abundant, cheap, and nontoxic, and there are other recent examples of it acting as a catalyst in the synthesis of valuable nitrogen heterocycles in DMSO.7,8 In fact, there has been another report recently of the synthesis of benzimidazoles using a copper catalyst in DMSO.5 (See “One Pot Benzimidazole Synthesis” in a previous post from February 5, 2012.)
It is noteworthy that DMSO out-performed all other solvents in the Punniyamurthy synthesis. For example, in the model reaction used for optimization the isolated yields dropped significantly with other solvents (Table 1).
Scale up of the procedure using 4-methylaniline and benzaldehyde (gram-scale) proceeded smoothly and resulted in a 73% isolated yield, so this reaction can be useful for preparing larger quantities.
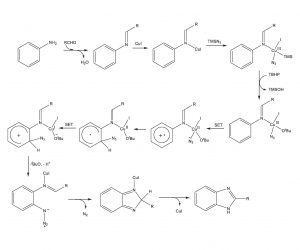
Mechanistic studies revealed 1) no kinetic isotope effect on replacement of the hydrogens ortho to the amino group in aniline with deuteriums, 2) disruption of the reaction with the addition of the radical scavenger TEMPO, and 3) ESI-MS analysis of the reaction mixture demonstrating four major species which could be derived from the mechanistic pathway indicated below.
Proposed Mechanism
In summary, the one-pot Cu(I)-catalyzed synthesis of substituted benzimidazoles from various anilines and aldehydes gives good to excellent yields in DMSO in most cases. The starting materials are readily commercially available and are generally inexpensive. The catalyst, CuI, is also inexpensive and readily available. This demonstrates another route to important nitrogen heterocycles through Cu-catalyzed chemistry in DMSO.
Their general procedure is described as follows.
Aniline (1.0 mmol) and aldehyde (1.2 mmol) were stirred at 60 oC for 1 h in DMSO (1 mL) under air. The mixture was then cooled to room temperature and treated with CuI (10 mol%, 0.1 mmol, 19 mg), TMSN3 (2 equiv, 2.0 mmol, 230 mg), and TBHP (1 equiv, 1 mmol, 181 µL). The resultant mixture was stirred at 90 oC for the appropriate time. The progress of the reaction was monitored by TLC using ethyl acetate and hexane as eluent. The reaction mixture was then cooled to room temperature and was extracted with ethyl acetate (3 X 10 mL) and washed with brine (2 X 5 mL) and water (2 X 5 mL). The solution was dried over Na2SO4, passed through a short pad of Celite, and evaporated on a rotary evaporator to give a residue that was purified on silica gel column chromatography using n-hexane and ethyl acetate as eluent.
Safety Note: Trimethylsilyl azide (TMSN3) is considered a safer alternative for delivery of the azide functional group than is hydrazoic acid. It will, however, hydrolyze to hydrazoic acid over time with exposure to water.
References
- Mahesh, D.; Sadhu, P.; Punniyamurthy, T. J. Org. Chem. 2015, 80, 1644.
- Carvalho, L. C. R.; Fernandes, E.; Marques, M. M. B. Chemistry – A European Journal 2011, 17, 12544.
- Baars, H.; Beyer, A.; Kohlhepp, S. V.; Bolm, C. Org. Lett. 2014, 16, 536.
- Nguyen, T. B.; Ermolenko, L.; Al-Mourabit, A. J. Am. Chem. Soc. 2013, 135, 118.
- Kim, Y.; Kumar, M. R.; Park, N.; Heo, Y.; Lee, S. J. Org. Chem. 2011, 76, 9577.
- Alla, S. K.; Kumar, R. K.; Sadhu, P.; Punniyamurthy, T. Org. Lett. 2013, 15, 1334.
- Debnath, P.; Majumdar, K. C. Tetrahedron Lett. 2014, 55, 6976.
- Jiang, X.; Sun, D.; Jiang, Y.; Ma, D. Tetrahedron Lett. 2015, 56, 3259.