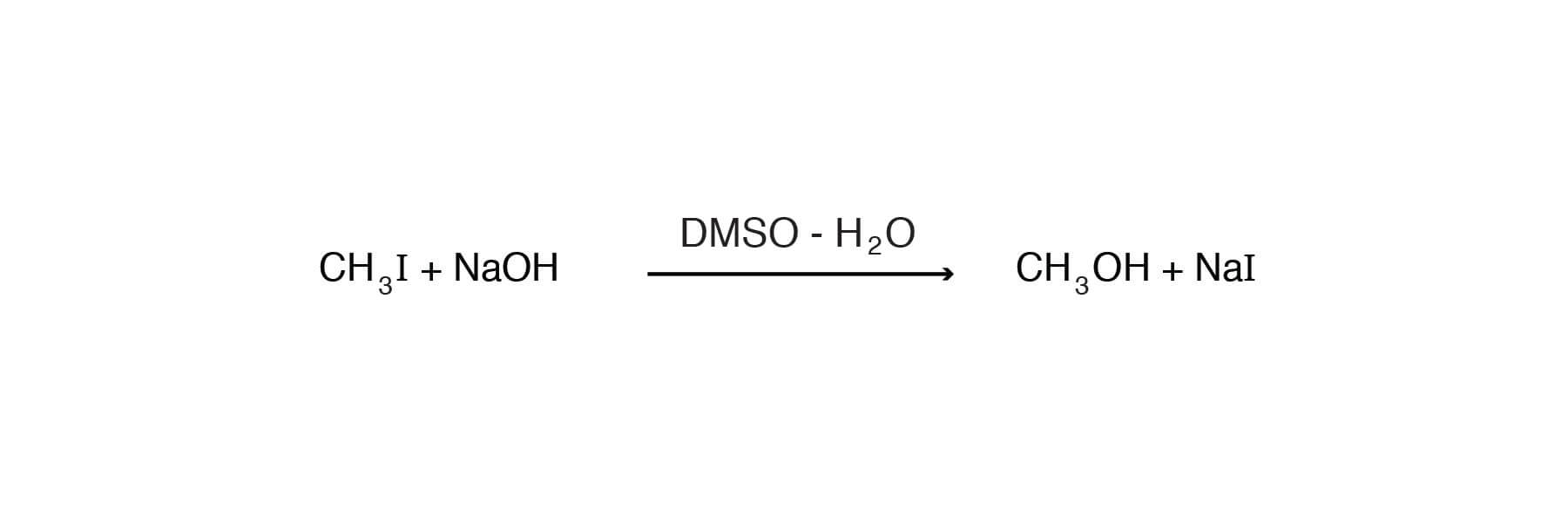
When the alkaline hydrolysis of methyl iodide is studied in the presence of hydroxyl ion in DMSO-water, the rate of hydrolysis increases with increasing DMSO content [Murto, J., Suomen Kemistilehti B34, 92-98 (1961)].
Similar results are obtained with other primary alkyl halides (iodides, bromides, chlorides)[Bockmann, T.; Haanaes, E.; Ugelstad, J. , Tidsskr. Kjemi. Bergv. Met. 24, No. 11, 209-215 (1964)]. The rate constants for the reaction of hydroxide ion with ring substituted benzyl chlorides in acetone-water and DMSO-water mixtures are reported as a function of both solvent composition and temperature. The reaction rate increases with increasing DMSO concentration but decreases with increasing acetone concentration [Tommila, E.; Pitkanen, I. P.,Acta Chem. Scand. 20, 937-945 (1966)].
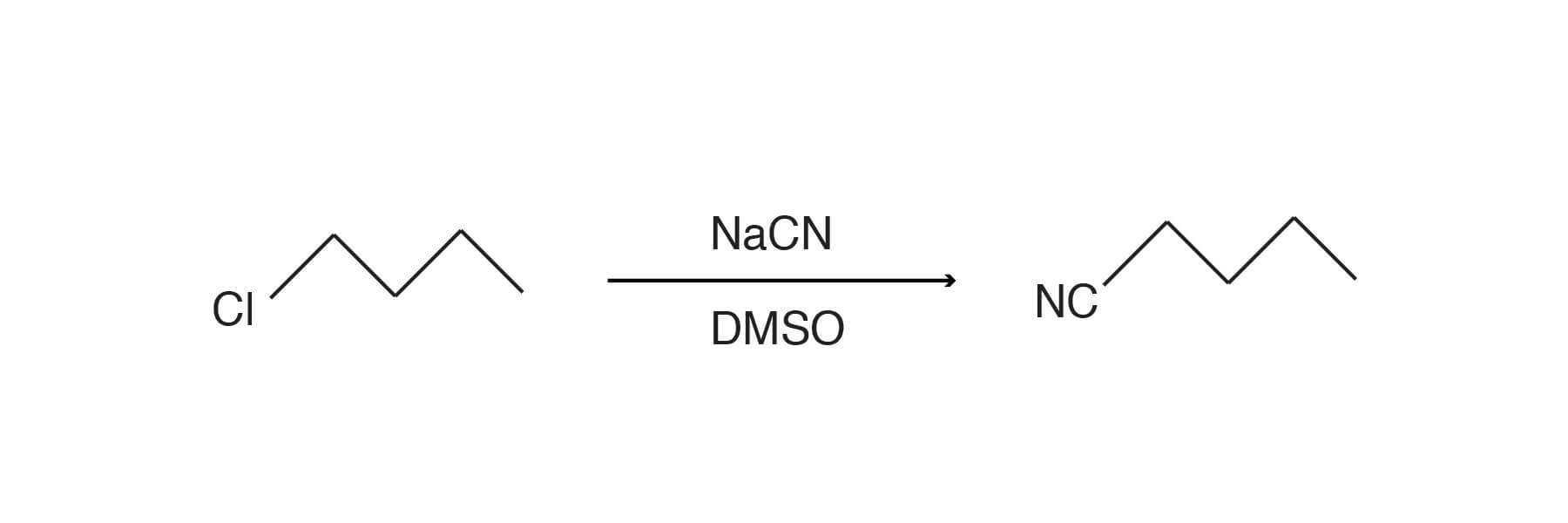
In another example, 1-chlorobutane (1 mole) was added over 15 minutes to a stirred suspension of NaCN (1.08 mol) in DMSO (250 ml) at 80°C. The mixture was cooled to maintain temp <140°C. After the addition was complete, the mixture was cooled and diluted with water to 1L. The aqueous mixture was extracted with ether (3 x 150 ml). The ether extracts were washed with 6N HCl, then water, dried over CaCl2, and evaporated yielding a residue which was distilled to provide the product in 93% yield. Adapted from J. Org. Chem., 25 1388 (1960).