The importance of the Wittig Reaction lies in the fact that this is a carbon-carbon bond-forming reaction, particularly useful for the synthesis of alkenes and polyenes. For example, the complex polyene beta-carotene has been synthesized by sequential Wittig reactions.
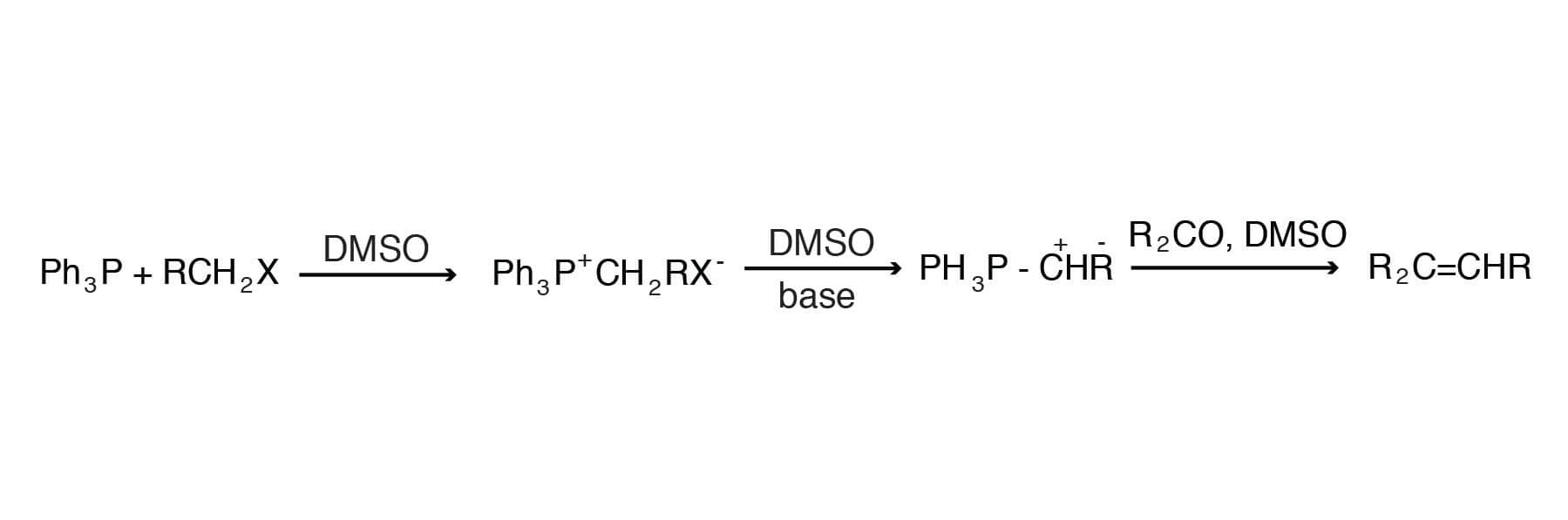
This reaction proceeds through the addition of phosphorus ylides to suitable carbonyls. The mechanism involves two sequential steps. The first typically involves de-protonation of a triphenylphosphonium halide, followed by addition of the intermediate ylide (also known as a phosphorane) to a carbonyl of an aldehyde or ketone. Elimination of triphenylphosphine oxide provides the alkene product.
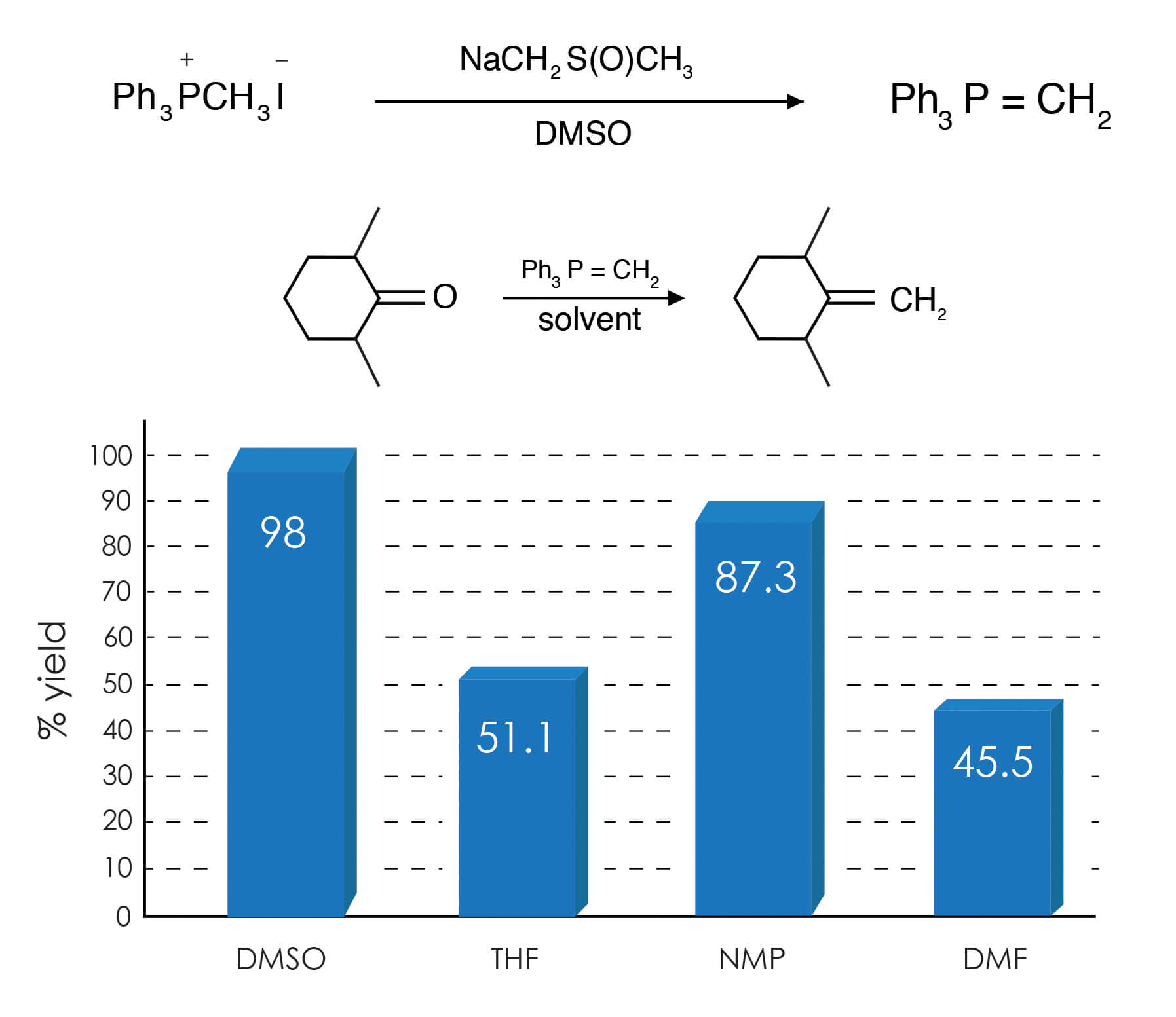
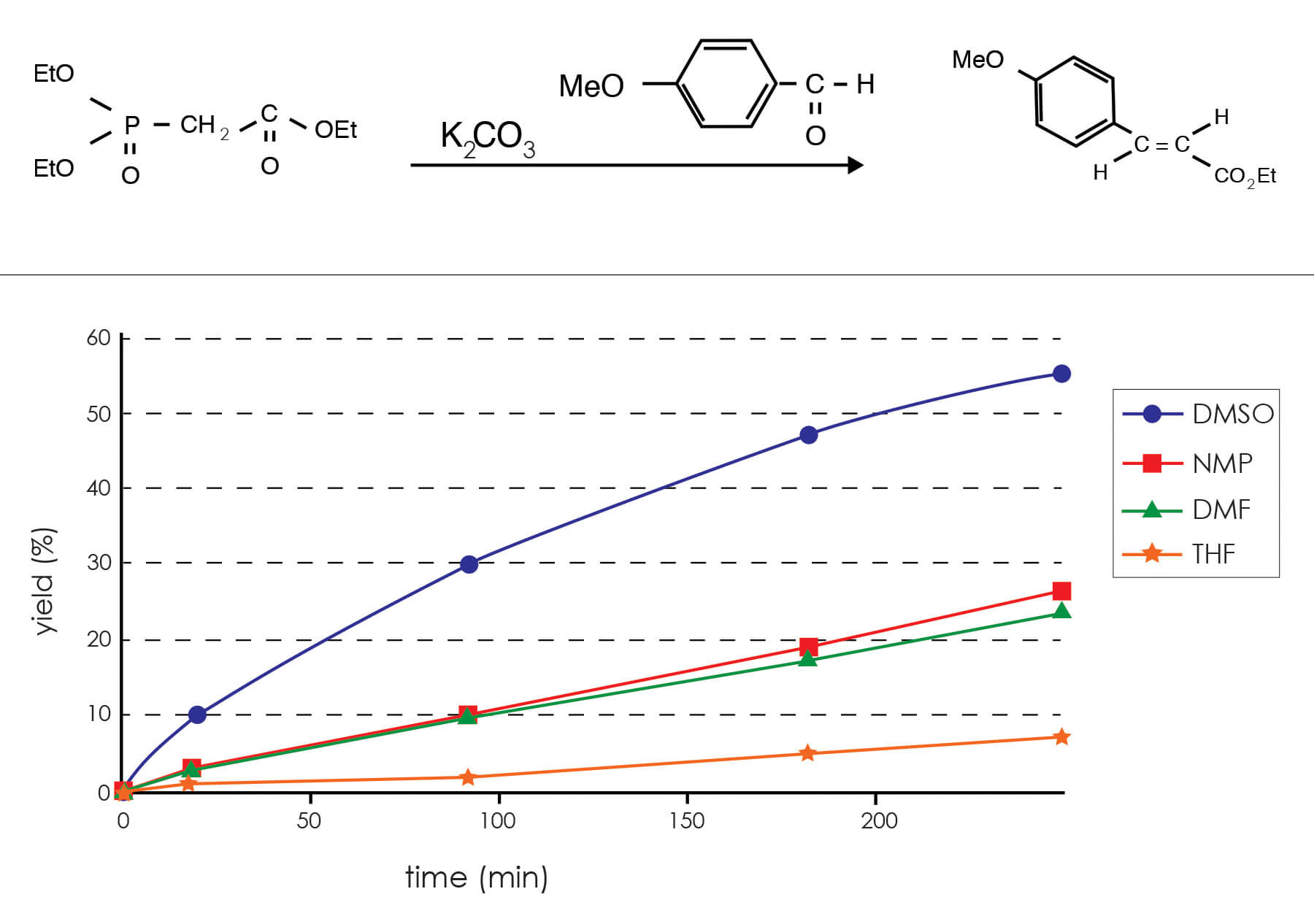
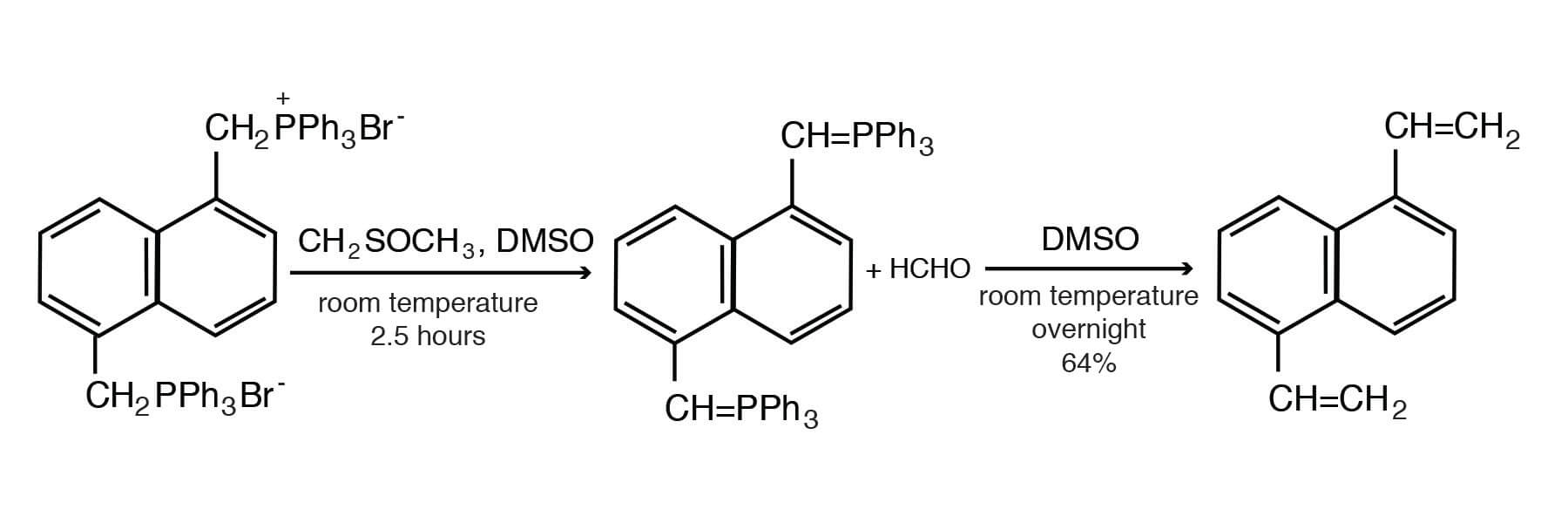
As mentioned above, the Wittig reaction converts carbonyl compounds to olefins. Thus, the reaction of formaldehyde and the phosphorane derived from 1 ,5-bis(triphenylphosphonomethyl)naphthalene dibromide in DMSO gives 1,5-divinylnaphthalene [Fleming, R. H.; Quina, F. H.; Hammond, G. S., J. Am. Chem. Soc. 96, 7738-7741 (1974)].