The development of methods to efficiently incorporate trifluoromethyl groups into compounds continues to be of interest to pharmaceutical, agrichemical, and material chemists. A recent report by the Hamashima group demonstrates the rapid, direct trifluoromethylation at the benzylic position of 2-methylbenzophenones and 2-methylacetophenones 1 using only the Togni reagent II (2) and photoirradiation (365 nm) in DMSO (Table 1).1
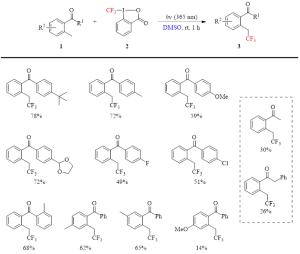
Table 1: Isolated yields in the photo-induced trifluoromethylation of 2-methylbenzophenones and 2-methylacetophenones by the Togni reagent II in DMSO.
In the optimization of the reaction conditions, it was found that DMSO significantly outperformed all other solvents. This is another example where DMSO is the preferred solvent for a photoreaction. (For other examples, see Synthesis Corner posts dated July 9, August 2, and October 1, 2018.)
As can be seen in Table 1, this reaction produces the trifluoromethylated product with generally good isolated yield after only one hour of photoirradiation at room temperature. It is site selective, with only the ortho methyl group being trifluoromethylated, even when other methyl groups are present on the benzophenone ring. Lower yields are encountered when R2 is the strongly electron-donating substituent of 4-MeO (final entry in Table 1), and when the R1 group is an alkyl group instead of an aryl group (entries in the dashed line box in Table 1).
It is believed that this reaction proceeds through a photo-induced enol of 1 which then attacks the electrophilic Togni reagent to produce the trifluoromethylated species 3. This ionic reaction mechanism is supported by the fact that the inclusion of TEMPO does not significantly diminish the yield, and no radical trapping products are detected.
To summarize, this group has developed a photo-induced reaction for the trifluoromethylation of 2-methylbenzophenones and 2-methylacetophenones at room temperature with only a trifluoromethylating agent, photoirradiation for one hour, and DMSO. No additives or transition metals are needed to achieve good yields. This reaction represents another example where yields for photochemical reactions are significantly improved by using DMSO as the solvent.
Debra D. Dolliver, Ph.D.
1Ide, T.; Masuda, S.; Kawato, Y.; Egami, H.; Hamashima, Y. Org. Lett. 2017, 19, 4452.


