Introduction
The manufacture of microelectronic devices uses a range of solvents in a variety of applications. Solvents are chosen based upon their properties to deposit coatings, remove photoresist and related polymers, and to carry out general cleaning activities. As the electronics industry continues to create new technologies, serious challenges exist in their efforts to improve cleaning, comply with safety regulations and to reduce cost. Dimethyl Sulfoxide (DMSO), a common solvent for many polymers, is a key solution to helping the industry meet their objectives. This bulletin provides pertinent information and guidelines in using DMSO in microelectronic applications.
Contents
- Process Objectives
- Investigation of DMSO in microelectronics
- Results of Investigations
- Conclusions and Applications
- Bibliography
- Appendix A: DMSO Properties
- Appendix B: Health Safety and Environmental Overview
- Appendix C: DMSO Material Compatibility
Process Objectives
When designing and integrating a new process in microelectronic manufacturing, there are key objectives which must be achieved. Generally the process must meet minimum (a) performance and (b) selectivity criteria, while maintaining (c) stability for extended periods of time and (d) compatibility with a wide range of materials pertinent to the tool. These are described in more detail below in Table 1 along with DMSO’s application information.
Table 1
Microelectronic Cleaning Processes and DMSO Benefits
| Objective | Cleaning Process Requirements | DMSO Benefits |
| Performance | Ability to exhibit high solvency to a wide range of polymers and residues, rinse in water and other solvents, leaving the substrate clear and free of residue. | A wide variety of organic compounds including polymers are soluble in DMSO. DMSO is completely miscible with water and lower alcohols. |
| Selectivity | Ability to protect substrate, metal, and certain dissimilar polymers through out the manufacturing process. | While acting as a strong solvent for polymers, DMSO is extremely mild on metals and oxides such as ceramics. |
| Stability | Ability for the system’s properties to not change over an extended period of time. The longer this time period, the more robust the process and opportunity for cost savings. | DMSO’s high degree of stability in the presence of acids and bases at elevated temperature produces cleaning formulations that are robust for manufacturing processes. |
| Compatibility | Ability for the system to be easily integrated into existing tool platforms and have little or no effect on the materials of construction. | Teflon® fluoropolymer, polypropylene, polyethylene, and many metals and alloys are suitable for DMSO contact in the tool. Other materials should be evaluated for compatibility. Please reference Appendix C for a list of compatible materials. |
| Safety | Products deemed to be safe do not exhibit characteristics that present an acute or chronic hazard to workers or the equipment. | DMSO is safe to humans and biodegradable in the environment. The material can be recycled using distillation technology. Additional Health, Safety and Environmental information is included in Appendix B. |
DMSO Investigation
Dimethyl Sulfoxide exhibits properties which make it attractive in manufacturing. To better understand these benefits, Gaylord Chemical has conducted an investigation of DMSO, and compared it to many other solvents for microelectronic applications. Common polymers known to be used in electronics were included in this investigation (See Table 2). The polymers were tested for their solubility in DMSO and other solvents.
Table 2
Polymer Samples Used for DMSO Benefits Investigation
| Product | Application | Polymer Type | |
| A | Wafer thinning spinon adhesive | Wafer level packaging | TPU (Estane™ 5703,Noveon) |
| B | Neg PR (i-line) | WLP Bumping | Acrylic (Dianal ™ ACP-3, Dianal America) |
| C | Wafer thinning spinon adhesive | Wafer level packaging | Rosin (Resinall ™ 833, Resinal Corp.) |
| D | Dielectrics | WLP Insulator | imide-based (Torlon™ 4000T, Solvay) |
| E | Positive PR (DUV) | IC lithography | PHOST |
| F | Positive PR (i-line) | FPD, IC lithography | novolac |
Over twenty (20) solvents were surveyed for solvency, blended together and mixed with acids and bases at different temperatures. These solvents were selected based on their common occurrence in photoresist stripping, casting, and rinse applications. They include amides, glycol ethers, lower alcohols, esters, ketones, and lactones. Although many of these solvents may exhibit good performance, very few provide the same benefits, in total, of DMSO. These benefits include: high flash point, high polarity /dielectric constant, water solubility, and low toxicity.
The acid and base classes tested in the study included alkylammonium hydroxides, alkanolamines, amines, and sulfonic acids. These are well known candidates in microelectronic formularies. These aggressive materials are needed to break up and solvolyze long chain polymers complex which may be implanted with metals. Hard, baked polymers with impregnated metal are a common occurrence in many deposition or etch processes.
Results
For ease of observation and to identifying key trends, dissolution rates of the noted polymers identified in Table 2 are assigned shades. The highest solubility is indicated by light blue, limited or no solubility is identified in dark red, and partial solubility is dark blue. Table 6 provides details on the solvents referenced in Tables 3-5.
1. General Polymer Solubility
DMSO exhibits excellent solubility for polymers used in microelectronic manufacturing as a complement to existing and common process of record (POR) solvents (see Table 3). Item #1 (DMSO), #2 (pyrrolidone), #5 (amide) and #19 (ketone) all exhibit excellent solubility at a temperature.
Table 3
Dissolution of Polymers at 70˚C by Solvents
Commonly Used in Microelectronics
| Estane 5703 | Dianal ACP-3 | Reinall 833 | Torion 4000T | Phost | Novolac | |
| DMSO | ||||||
| NMP | ||||||
| EL | ||||||
| BLO | ||||||
| DMAc | ||||||
| EG | ||||||
| THFA | ||||||
| FAM | ||||||
| PGMEA | ||||||
| MAK | ||||||
| PM | ||||||
| DB | ||||||
| DPM | ||||||
| NEP | ||||||
| HEP | ||||||
| CHP | ||||||
| 2-pyrol | ||||||
| MA | ||||||
| acetone | ||||||
| IPA | ||||||
| EB |
| Soluble | Partially | Insoluble |
2. DMSO Blends
The addition of DMSO as a cosolvent to an existing chemistry is expected to increase solvency for those polymers tested. The results in Table 4 (DMSO Blends) reflect an almost complete improvement from that observed in Table 3 (single solvent testing).
Table 4
Dissolution of Polymers by DMSO – Solvent Blends
(1:1 = 50% DMSO)
| Estane 5703 | Dianal ACP-3 | Reinall 833 | Torion 4000T | Phost | Novolac | |
| DMSO | ||||||
| NMP | ||||||
| EL | ||||||
| BLO | ||||||
| DMAc | ||||||
| EG | ||||||
| THFA | ||||||
| FAM | ||||||
| PGMEA | ||||||
| MAK | ||||||
| PM | ||||||
| DB | ||||||
| DPM | ||||||
| NEP | ||||||
| HEP | ||||||
| CHP | ||||||
| 2-pyrol | ||||||
| MA | ||||||
| acetone | ||||||
| IPA | ||||||
| EB |
| Soluble | Partially | Insoluble |
3. Solvency with Additives
The solubility of the polymers tested is maintained in DMSO even after addition of base. The observation of Table 5 and comparison with the baseline dissolution results for pure solvents in Table 3 suggest that many solvents result in a reduced solvency upon addition of base
Table 5
Dissolution of Polymers by DMSO with 20%
Added Alkanolamine, at Room Temperature
| Estane 5703 | Dianal ACP-3 | Reinall 833 | Torion 4000T | Phost | Novolac | |
| DMSO | ||||||
| NMP | ||||||
| EL | ||||||
| BLO | ||||||
| DMAc | ||||||
| EG | ||||||
| THFA | ||||||
| FAM | ||||||
| PGMEA | ||||||
| MAK | ||||||
| PM | ||||||
| DB | ||||||
| DPM | ||||||
| NEP | ||||||
| HEP | ||||||
| CHP | ||||||
| 2-pyrol | ||||||
| MA | ||||||
| acetone | ||||||
| IPA | ||||||
| EB |
| Soluble | Partially | Insoluble |
Unlike most solvents, the results of Table 5 suggest that candidate solvents #1 (DMSO), #2 (pyrrolidone), and #5 (amide) will maintain their solvency for the noted polymers after addition of base. Many systems exhibit significant reduction in solvency (e.g. item #’s 9-14=glycol ethers and acetates).
| Solvents | CAS# | Common Name |
| DMSO | 67-68-5 | dimethyl sulfoxide |
| NMP | 872-50-4 | N-methyl pyrollidone |
| EL |
97-64-3 |
ethyl lactate |
| BLO |
96-48-0 |
butyrolactone |
| DMAc |
127-19-5 |
dimethylacetamide |
| EG |
107-21-1 |
ethylene glycol |
| THFA |
97-99-4 |
tetrahydrofurfuryl alcohol |
|
FAM |
75-12-7 |
formamide |
| PGMEA |
108-65-6 |
propylene glycol methyl ether acetate |
| MAK |
110-43-0 |
methyl isoamyl ketone |
| PM |
107-98-2 |
propylene glycol methyl ether |
| DB |
112-34-5 |
diethylene glycol butyl ether |
| DPM |
2687-91-4 |
dipropylene glycol methyl ether |
| NEP |
2687-91-4 |
n-ethyl pyrollidone |
| HEP |
11/2/3445 |
hydroxyethyl pyrollidone |
| CHP |
6837-24-7 |
cyclohexyl pyrollidone |
| 2-pyrol |
616-45-5 |
2-pyrollidone |
| MA |
79-20-9 |
methyl acetate |
| acetone |
67-64-1 |
|
| IPA |
67-64-0 |
isopropyl alcohol |
| EB |
111-76-2 |
ethylene glycol butyl ether |
| DE |
111-90-0 |
diethylene glycol ethyl ether |
4. Stability with Aggressive Additives
DMSO exhibits excellent stability as a process solvent in the presence of aggressive additives such as strong bases. Many common solvents react with bases such as alkylammonium hydroxides and amines.
Stability testing was performed by comparing pH changes in DMSO formulations with other solvent blends. Accelerated stability testing with DMSO/alkylammonium hydroxide formulations showed no change in pH after 24 hours at 80 ̊C.The two leftmost formulations in Figure 1 are DMSO-based products. It is worthwhile to mention that these formulations do not exhibit a color change during the test period.

Figure 1. Stability tests comparing basic DMSO formulas (Left vials = clear solutions) to other solvent blends
In high pH formulations with amides and certain pyrrolidones appear to be especially sensitive to base exposure. Mixtures dropped at least 2 pH units suggesting a chemical reaction between the solvent system and the base.
5. DMSO in Microelectronic Applications
Test results suggest DMSO exhibits significant benefits in photoresist (PR) stripping from microelectronic devices. Information from the polymer dissolution work in this investigation indicates that DMSO is a good choice for common novolac and PHOST PR stripping (see Tables 2-4). Stripping of a negativetone PR3 may require the addition of base to facilitate bond- breaking and solvolysis. DMSO is a good choice as a high solvency medium with good stability when mixed with bases (compare Tables 3 & 5 and reference Figure 1). To this end, we have confirmed DMSO’s performance on sample microelectronic devices noted in Figures 2 & 3.
Conclusions and Applications
DMSO has been investigated for its solvency and other performance-based properties that are key indicators of its ability to solve problems in the microelectronics industry. The following are general conclusions from these studies:
- DMSO is an excellent solvent for common polymers used in microelectronics.
- The addition of DMSO is expected to improve solvency of other solvents.
- Additives such as alkanolamines are not expected to diminish the solvent
- performance of DMSO.
- DMSO exhibits good stability when combined with aggressive materials such as strong bases.
- DMSO has been demonstrated to strip conventional novolac and negative tone acrylic PR from microelectronic devices.
TFT-LCD photoresist stripping applications. DMSO is a known formulation solvent for stripping novolac resist materials used in TFT-LCD flat panel displays. DMSO/amine and DMSO/alkylammonium hydroxide formulations have become industry standards.
High Dose Implanted (HDI) resists. The patterning conditions used to make sophisticated IC devices has a hardening influence on photoresist residues. Prior to stripping, a resist may be dosed with dopants, during an aggressive heated process. Strong solvents and aggressive stripping media are needed to safely remove carbonized resists from silicon and related substrates. The high polarity of DMSO makes it an attractive component of HDI resist residue cleaners.
Coating Solvent Applications. Wet photoresist formulations are polymer solutions, which are typically spun onto a substrates. One or more baking steps are used to drive off the formulation solvent to produce a resist film appropriate for lithographic patterning. DMSO may be used as a cosolvent in resist formulations when formulation stability requires the use of polar additives.
Wafer Level Packaging Applications. Lithographic processes using acrylic dry-film resist products6 are being used increasingly in chip-scale packaging applications. DMSO is a useful cleaning solvent for stripping these materials after bump patterning. There are two dominant wafer-bumping processes in practice today, Flip-Chip and C4. Each process flow is shown in Figure 6. The different conditions experienced by resist materials in both processes has an impact on the formulation of cleaning/stripping products for these processes.
Flip Chip processes often use acrylic dry film resist products, while the C4 process uses wet resists, with electroplated solder (novolac PR strip) and Figures 4 & 5 (negative tone acrylic PR strip).
Photoresist stripping – TFT-Array Patterning. A single DMSO-based formula was demon- strated to work well in stripping novolac PR from specific areas on a TFT-LCD device (Figures 2 & 3).
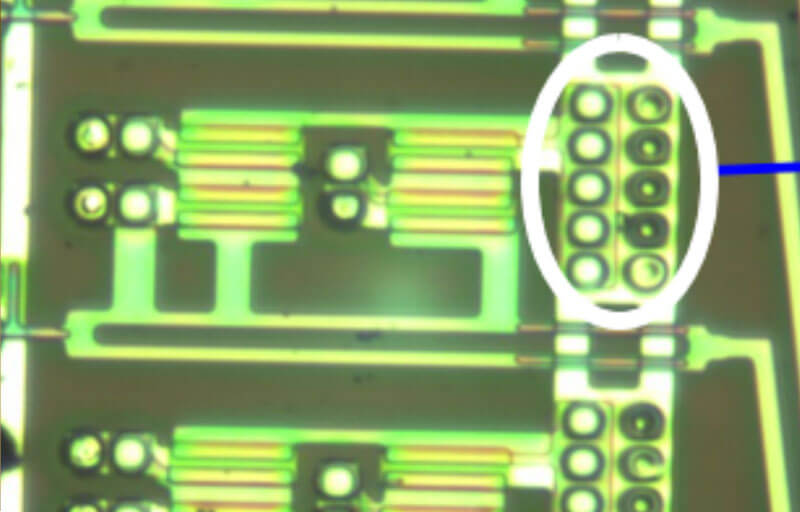
Figure 2. Area on device showing the presence of PR before cleaning.

Figure 3. Area on device following PR stripping with a DMSO- based chemistry.
Photoresist Stripping – Solder Bump Patterning4. In another microelectronic application, a DMSO based formula was prepared with an base used to strip and dissolve negative-tone acrylic PR. This PR was used to pattern and define metallized areas that remained for solder bumping. The optical microscope photos in Figures 4 & 5 show the thick PR in a before condi- tion and following exposure with the DMSO chemistry. (i.e. 80˚C, 30 min).
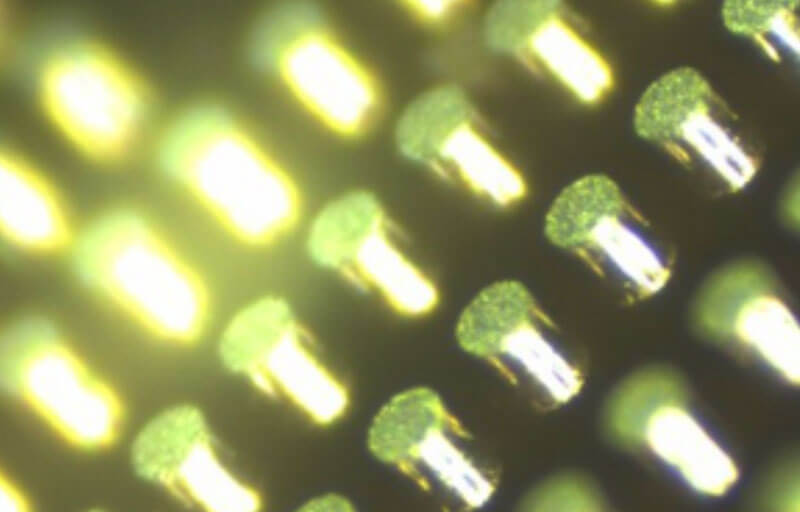
Figure 4. Area on the device showing the before condition of the top surface of the solder metal surrounded by cured thick PR. (50x)

Figure 5. Area on the device following stripping of the thick PR with DMSO/base mixture. (500x)
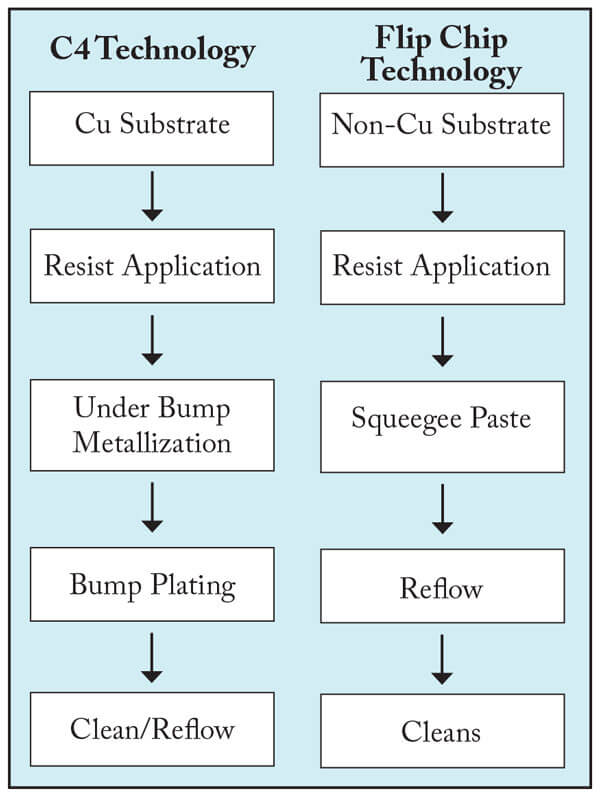
Figure 6. General process flow for bumping operations
Reflow is typically done in the resist pattern for Flip-Chip processing while the C4 process will remove the resist prior to reflow.
In the Flip-Chip process, the resist material is hardened requiring more aggressive DMSO- based formulations to remove photoresist and post-strip residue.
Rework Applications. DMSO has proven applications in solvent formulations to rework defective microelectronic components. These applications include removal of polyimide films used in TFT-LCD color filter applications and finished wafer passivation films. DMSO may also be useful in printed circuit rework applications.
Photoresist Stripping – Semiconductor Device Manufacturing. DMSO is a component of photoresist stripping products which are compatible with silicon substrates.
Wafer-Thinning Adhesives. The back lapping process uses adhesive materials to hold GaAs wafers for grinding. DMSO is useful in removing adhesive materials when the process is complete.
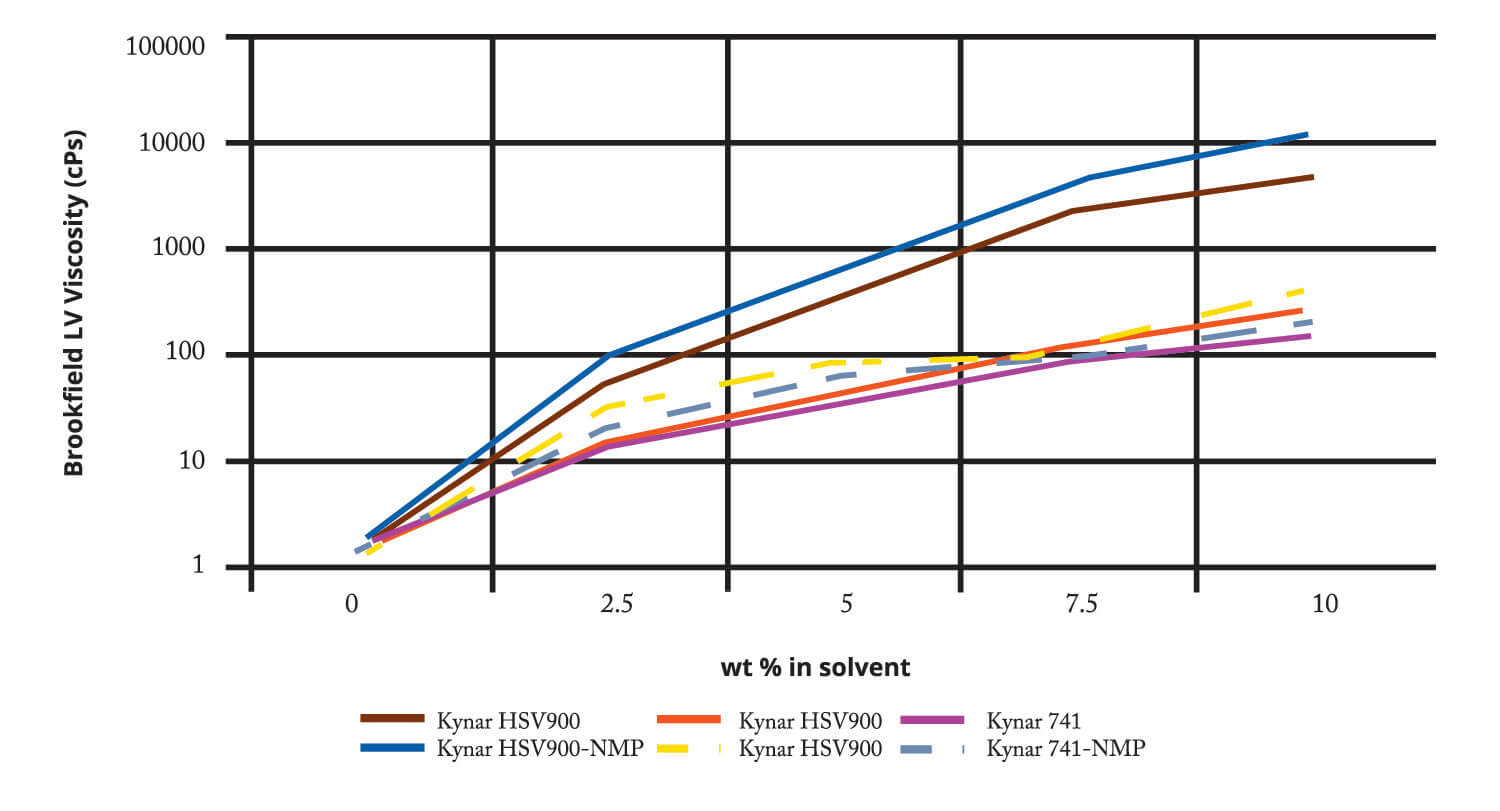
Secondary Lithium Ion Battery Manufacturing. DMSO has been reported as a solvent for lithium salt electrolytes and as a solvent for PVDF binder in batteries. Below is data comparing the solubility of PVDF products used in the battery industry in DMSO and NMP. Solubilities of Kynar™ PVDF in both solvents are similar, with similar solution viscosities.
Comparative Solution Viscosities:
Kynar PVDF in DMSO and NMP (25C)
Bibliography
- Lan, J. Low Cost Flip-Chip Technologies, Ch. 10, The McGraw-Hill Companies, Inc., New York, NY, 2000.
- Totta, P.A. and Sogher, R.P. IBM J. Res. Develop., 13, No. 3, 226 (1969).
- Shaw, J. ; Gelorme, J.D. ; LaBianca, N.C. ; Conley, W.E. and Holmes, S.J. “Negative Photoresists for Optical Lithography” 41 1/2 (1997)
- C4 description reference: a) US 5,904,156 May 18,1999 b) K.. Gilleo in Area Array Package Design, Ch. 1. The McGraw-Hill Companies, Inc., New York, NY, 2000.
- Mohondro, B. “Photoresist Application: An Art…and a Science” Microelectronic Manufacturing and Testing April 1990, p 56-57.
- Reiser, A. Photoreactive Polymers: The Science and Technology of Resists John Wiley and Sons, New York, NY, 1989, p168.
- Ward, I.E; Michelotti, F. Peters, D. U.S. Patent 5,698,503, 1997.
- Hua, Chang-Hwang; Day, D.Y. U.S. Patent 5,127,984 1992 b) Nishiguchi, M. ; Goto, N. Ni- shizawa H. J. Electrochem. Soc. 138, 6 (1991) p1826-1830 c) Grupen-Shemansky, M U.S. Patent 5,268,065, 1992.
- Lee, Y.; Kim, K.; Ryu, K.; Chang, S. US Patent Appln 20050196677 (2005) ; Filler, R.;
- Luo, K. Mandal, B. “Synthesis and Characterization of Novel non-fluorinated Tri-lithium Imide Salts for Use in Lithium-ion Battery Electrolytes” Solid State Ionics 177 V9,10 (2006) p857-861
- Coustier, F. “ Method of Fabricating a Laminated Battery Cell” “ WO 20000069010 (2000); Coustier, F. “Battery Cell Separator and Fabrication Process” WO 2002050929 (2002)
Appendix A
DMSO Properties
| Parameter | Value |
| Water Solubility | Miscible |
| Density, at 25°C (77°C) | 1.0955 g / cm3 |
| Viscosity at 20°C | 2.14 cP |
| Surface tension at 20°C | 43.53 dynes / cm |
| Refractive index n_Dˆ20 @20°C | 1.4785 |
| Dielectric constant, 1 MHz, @ 20°C | 48.9 |
| Dielectric constant, 1 MHz, @ 40°C | 45.5 |
| Conductivity (Electrical) @ 20°C | 3×10-8 (ohm-1 cm-1) |
| Conductivity (Electrical) @ 80°C | 7×10-8 (ohm-1 cm-1) |
| pKa | 35.1 |
| pH (50% in water) | 8.5 |
| Boiling Point | 189°C (372°C ) |
| Flash point (open cup) | 95°C (203°F) |
| Flash point (closed cup) | 89°C (192°F) |
| Freezing point | 18.55°C (65.4°F) |
| Autoignition temp, in air | 300-302°C (572-575°F) |
| Heat capacity (liq.), 25°C | 0.47 cal / g / °C |
| Solubility Parameters | |
| Hildebrand’s | 13.0 (cal / cm3)1/2 |
| Hansen’s | |
| – Dispersion | 9.0 (cal / cm3)1/2 |
| – Polar | 8.0 (ca l / cm3)1/2 |
| – Hydrogen bonding | 5.0 (cal / cm3)1/2 |
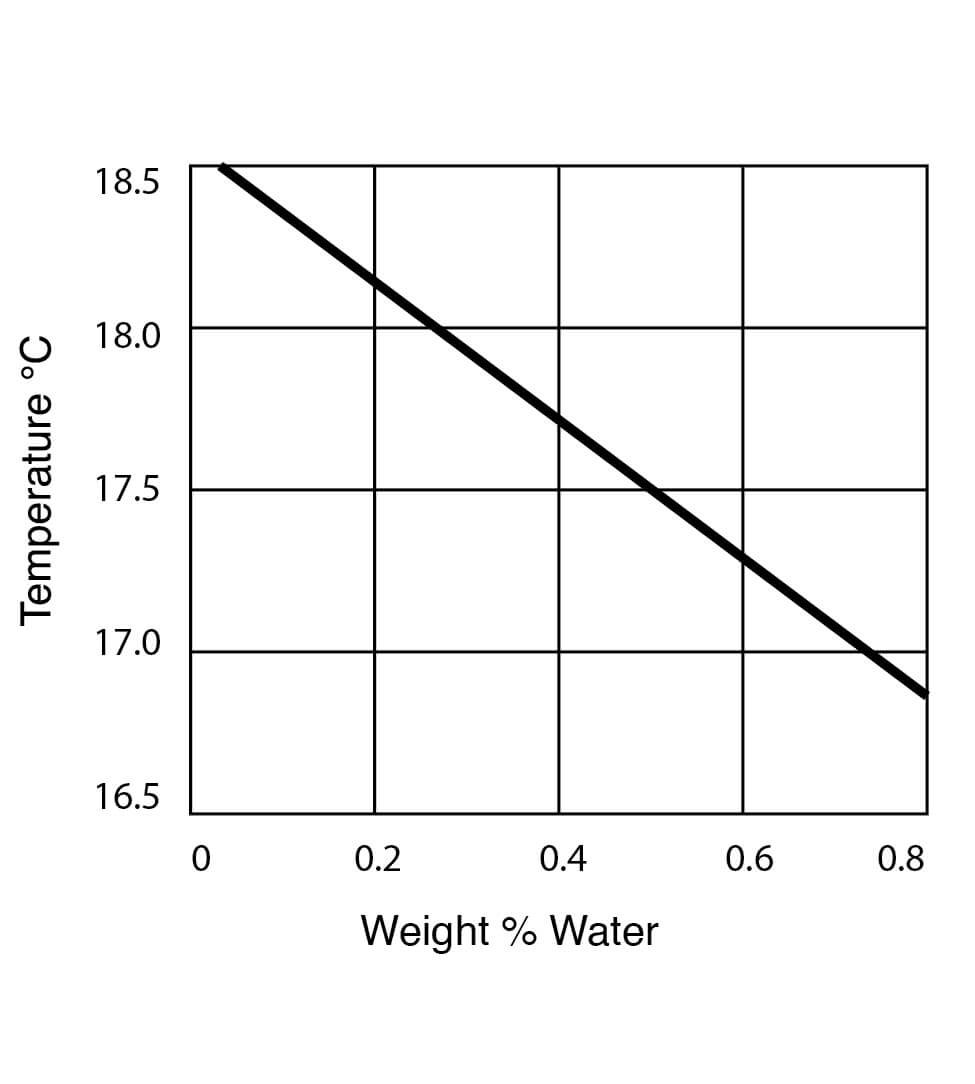
Freezing Point Data for DMSO Water Solutions
Figure 9
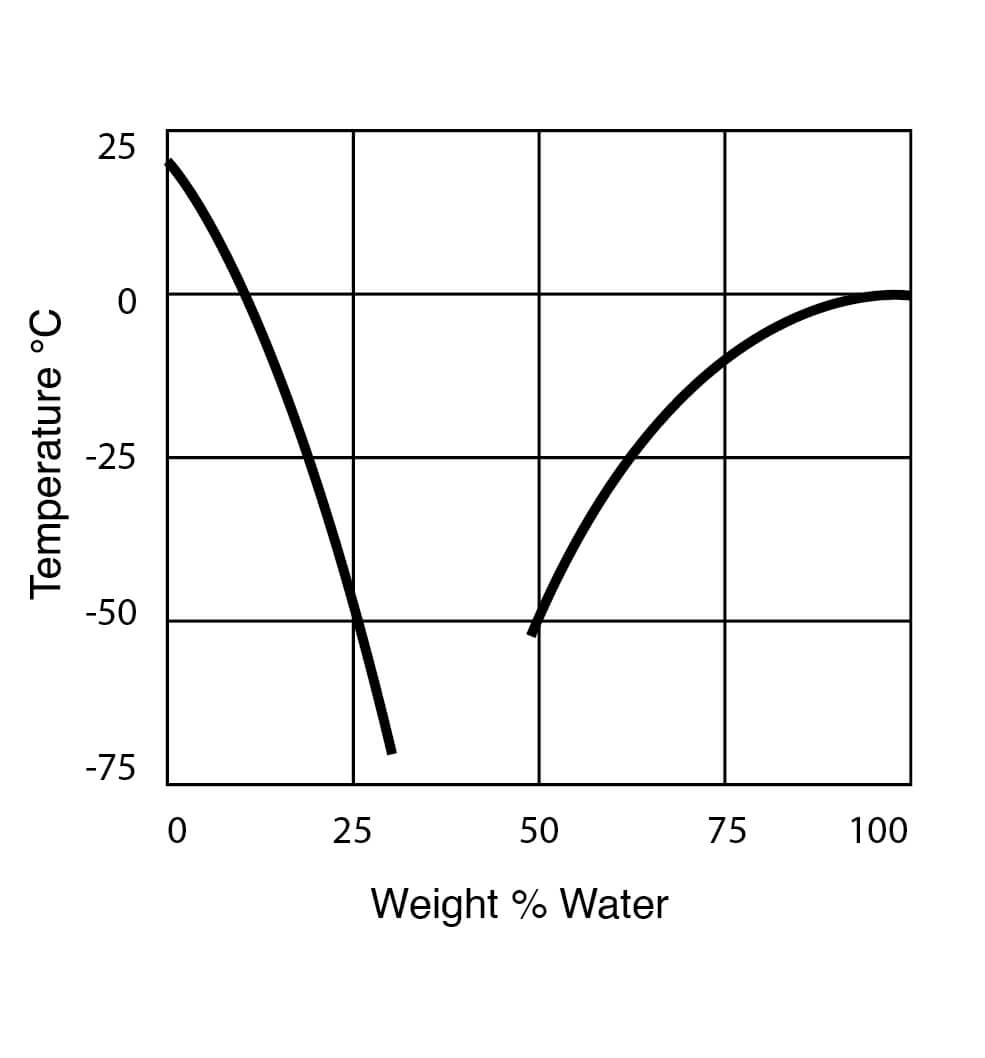
Figure 10
Appendix B
Freezing Point Data for DMSO Water Solutions
Health (with comparison to other solvents)
| Toxicological Indicator | DMSO | DMAc | NMP |
| Oral LD50 | 14,500-28,300 mg/kg rat | 4,300 mg/kg rat | 3,914 mg/kg rat |
| dermal LD50 | 40,000 mg/kg rat | 2,240 mg/kg rat | 8,000 mg.kg rabbit |
| Inhalation (rat) | none @ 2,900 mg/m3 | 2,475 mg/kg @1 hr | NA |
| Reproductive Toxin | no | yes | yes |
| Proposition 65 | no | no | yes |
Safety
Personal Protection: should include wearing butyl rubber or nitrile (NBR) rubber gloves and tight fitting safety goggles for eye protection. A recommended product is the TNT™ Disposable Nitrile Glove produced by Ansell Healthcare (VWR cat # 32889-888)
Use and Handling: Avoid contact with skin, eyes and clothing. Do not use near heat or open flame, distill with caution.
Storage conditions: Keep container closed, store away from heat and light.
Temperature Parameters
| Autoignition temp, in air | 300-302°C (572-575°F) |
| Flash Point (open cup) | 95°C (203°F) |
| Flash point (closed cup) | 89°C (129°F) |
| Flammable Limits (% in air): | LEL: 3.0-3.5% by volume |
| Flammable Limits (% in air): | UEL: 42-63% by volume |
Environmental
DMSO is biodegradable in biological systems, it is converted in part to methyl sulfone and to dimethyl sulfide.
Biological Oxygen Demand:
- Theoretical Oxygen Demand at 10 ppm: 123 mg oxygen
- Chemical Oxygen Demand at 10 ppm: 107 mg/1
- Biological Oxygen Demand-5 at 10 ppm: <1.0 mg/1
Note: For complete information please refer to the MSDS.
Appendix C
DMSO Material Compatibility
| Material | Solubility Grams/100cc DMSO | Solubility Grams/100cc DMSO |
| 20° – 30°C | 90° – 100°C | |
| Calcium Methanesulfonate | Soluble | |
| Diethanolamine | Miscible | |
| Ethyl alcohol | Miscible | |
| Methanesulfonic acid | Miscible | |
| Phosphoric acid | Miscible | |
| Rosin | > 100 | – |
| Triethanolamine laurylsulfate | Soluble | |
| Triethanolamine | Miscible | |
| Triethylamine | 10 | – |
| Polymers | ||
| Teflon® (DuPont) | Insoluble | Insoluble |
| Methacrylate’s Lucite 41, 45 (DuPont), Plexiglas (Rohm & Haas) |
– | < 1 |
| Phenoplasts Modified Novalac R7522, R7550 (Ceca) |
Soluble | – |
| Polycarbonates Lexan (General Electric) |
– | > 5 |
| Silicones Dow Corning “Sylkyd 50”, Z6018 (flake) |
Miscible | |
| Neoprene | Insoluble | Insoluble |
| Polyetherether ketone (PEEK) | Insoluble | Insoluble |
| Polyethylene | Insoluble | Insoluble |
| Polypropylene | Insoluble | Insoluble |
The information in this bulletin is based on information available to us and on our observations and experiences. However, no warranty is expressed or implied regarding the accuracy of this data, the results to be obtained from the use thereof, or that any use will not infringe any patent. Each user must establish appropriate procedures for off-loading, handling, and use of the product(s). Since conditions for use are beyond our control, we will make no guarantee of results, and assume no liability for damages incurred by off-loading, handling, or use of the product(s). Nothing herein constitutes permission, or recommendation to practice any invention covered by any patent without license from the owner of the patent.







