A breakthrough in the preparation of carbonyl compounds from alcohols was achieved with the development of reagents based on DMSO [Haines, A. H., Chem. Ind. (London), 883-887 (1976); (Epstein, W. W.; Sweat, F. W., Chem. Rev. 67(3), 247-260 (1967); (Durst, T., Advan. Org. Chem. 6, 285-388 (1969) CA 72, 21221Z.; Tidwell, T. T., Synthesis, 857-70 (1990)]. Several procedures have been developed which permit the selective oxidation of structurally diverse primary and secondary alcohols to the corresponding carbonyl compounds, i.e. aldehydes and ketones, respectively. The Swern Oxidation of alcohols avoids the use of toxic metals such as chromium, and can be carried out under very mild conditions. Most of these reactions take place at room temperature or above. Nucleophilic attack occurs on the DMSO sulfur atom. Most reactions in which the nucleophilic attack takes place on sulfur are aided by prior electrophilic attack on the oxygen atom [Mancuso, A.J.; Swern, D., Synthesis, 165-185 (1981)].
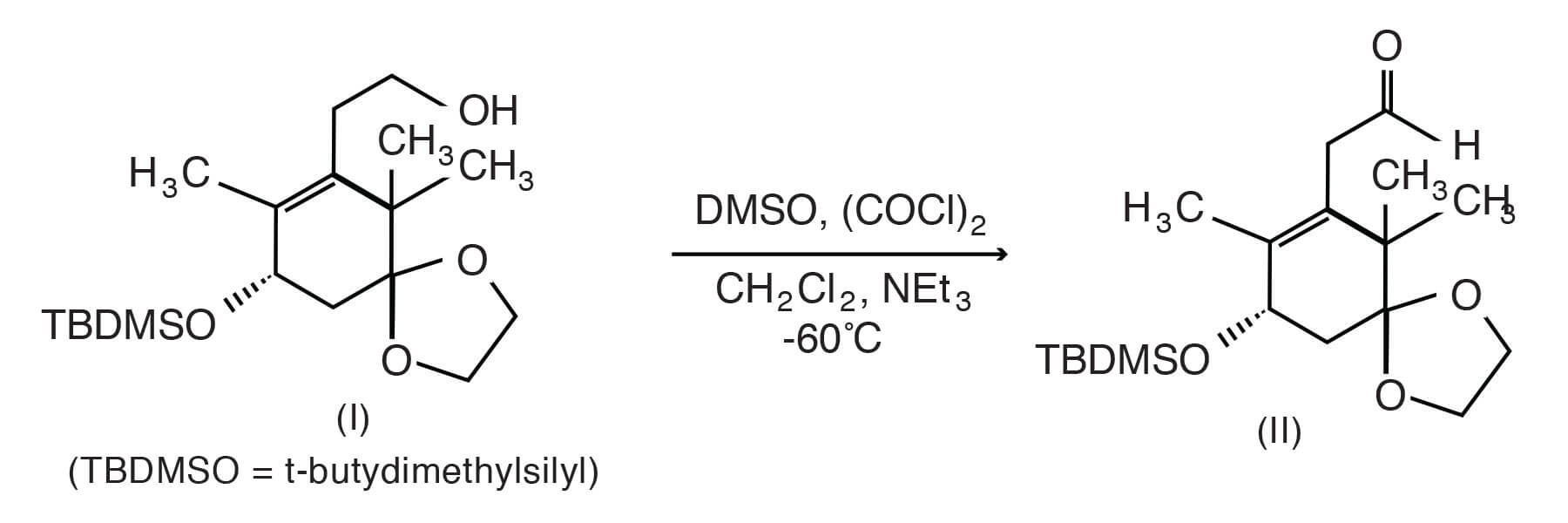
There are many examples of the Swern oxidation in contemporary literature. For illustrative purposes we have chosen a well-known compound, paclitaxel, whose synthesis includes a traditional Swern preparation in the oxidation reaction shown below. [Danishefsky; Samuel J., et. al., U. S. 5,416,225 (May 16, 1995)]. Thus, Swern oxidation of the primary alcohol of the TBDMS-protected compound (I) at -60°C in 15 minutes yielded, after treatment with triethylamine and warming to room temperature, the aldehyde (II), a key intermediate to paclitaxel.