Epoxides are ethers, also called oxiranes, in which the oxygen atom is contained in a three membered ring. This highly strained molecule is very reactive. The most commonly encountered reactions of epoxides are those in which the ring is opened by a nucleophile. Such reactions are advantageously performed in DMSO because DMSO is inert to the epoxides and it also provides maximum reactivity for the nucleophile. When the relatively unreactive 1-phenylcyclohexene oxide is heated with potassium hydroxide in aqueous DMSO, the corresponding trans-glycol is obtained in good yield [Berti, G.; Macchia, B.; Macchia, F.,Tetrahedron Lett. 38, 3421-3427 (1965)].
Epoxides are important chiral building blocks in organic synthesis.
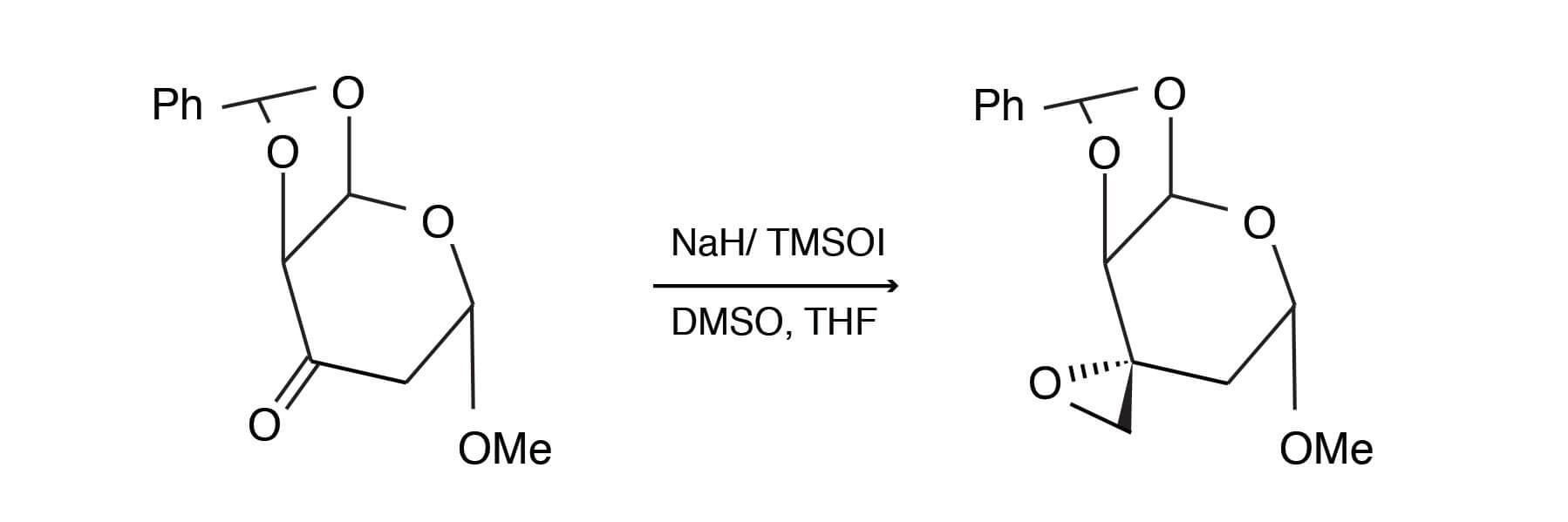
Sodium hydride (1.5 equiv) was suspended in DMSO/THF (3.0 ml of a 2:1 mixture). The suspension was stirred for 15 minutes. Trimethylsulfoxonium iodide (TMSOI), 1.5 equivalents was added, followed after 15 minutes, with 1.5 equivalents of substrate. The reaction was stirred until complete. Methylene chloride was added, followed by a typical extractive workup. The product was obtained in 81% yield using chromatographic purification. [Adapted fromLiebigs Ann. Chem., 1994, 999-1004].







