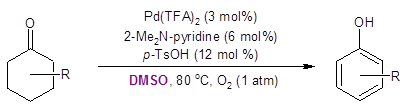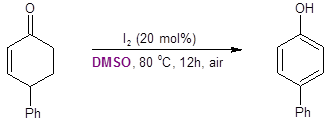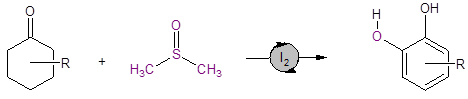
Equation 1: DMSO/I2 catalyzed route to substituted catechols
DMSO plays several critical roles in this reaction: it is the solvent, hydrogen acceptor, and oxygen source for the reaction. This route provides a streamlined synthesis of industrially and pharmaceutically important catechols.
The industrial/economic importance of catechols is substantial. As of 2005 there were greater than 300,000 biologically-active and pharmaceutically-useful compounds containing the catechol group, and greater than 3.0 X 107 kg of catechols were produced industrially each year at that point.2
Despite the importance of this moiety, classic synthetic techniques have been limited. These have generally begun with phenol and have required at least two steps. One route requires the ortho-formylation of phenol followed by the Dakin oxidation. Another requires the oxidation of phenol to an ortho-quinone followed by a reduction step. These methods also often have low regioselectivity with the installation of the new oxygen occurring ortho, meta, or para to the phenolic oxygen, requiring a sometimes challenging separation of different regioisomers and thus a low yield of the desired ortho-substituted compound.
Recently, the Stahl group developed a palladium-catalyzed route for dehydrogenative aromatization of cyclohexanones to phenols (also run in DMSO, Equation 2). 3,4
Equation 2: Conversion of cyclohexanone to phenol.
This transformation demonstrated the utility of cyclohexanones as starting materials for aromatic compounds. The work highlighted in this Synthesis Corner Post by Jiao’s group (Equation 1) adds to the attractiveness of cyclohexanone as a starting point for the synthesis of aromatic compounds.
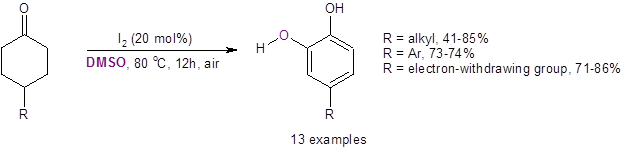
After optimization of the reaction conditions (20 mol % I2, DMSO, 80 oC for 12 h under air), the Jiao group tested the reaction on a variety of substituted cyclohexanones. They found that the reaction was highly regioselective in producing only the ortho-dihydroxyl aromatic compound. No other regioisomers were produced. They also found that, in most cases with a substituent at the 4-position, the reaction produced good yields of the substituted catechol (Equation 3). This reaction also worked well on a gram scale with the reaction with R=Ph resulting in a 72% isolated yield.
Equation 3: Yields with mild electron-donating groups and electron-withdrawing substituents at the 4-position.
With a strong electron-donating group (-OH, -OMe, or –OPiv) at the 4-position, however, the unsubstituted catechol was formed through an apparent 4-oxygen-substituent elimination. This was thought to be due to a reverse Michael addition reaction.
The reaction was also successful with electron-withdrawing substituents at the 3-position [3-COOH (63%) and 3–COOEt (53%)]. This reaction was found to be highly regioselective, only producing the 4-substituted catechol. (No 3-substitution was seen.)
While the reaction with a substituent at the 2-position did work, yields of the 3-substituted catechol product were low [2-CH3 (16%), 2-Bn (21%), 2-Ph (23%)].
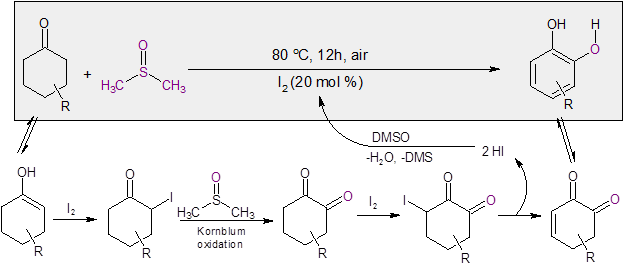
The researchers noted that the reaction proceeded under an argon atmosphere, indicating that the oxygen source was most likely from DMSO. Additional studies with 18O labeled DMSO substantiated this, with the labeled oxygen being incorporated in the catechol. Additional mechanistic probes indicated that iodination at the alpha position of the cyclohexanone followed by Kornblum oxidation appeared likely for this reaction.
With these findings, the following mechanism was proposed (Scheme 1).
Scheme 1: Mechanism for the I2/DMSO catalyzed formation of catechol.
Interestingly, the Jiao group found that when starting from 4-phenylcyclohexenone instead of 4-phenylcyclohexanone, the reaction resulted in the production of the 4-substituted phenol instead of the catechol (Equation 4).
Equation 4: Cyclohexenone conversion to a phenol with DMSO/I2.
To better understand this observation, these researchers performed DFT calculations. They assumed that the alpha-iodination step was common with both substrates. Their calculations compared the energy barriers for the SN2-like Kornblum oxidation pathway where the oxygen of DMSO displaces the iodide versus an E2-like pathway where DMSO assists in the elimination of HI.
They found that in the transition state for the SN2-like Kornblum oxidation pathway was energetically lower in the cyclohexanone substrate; however, the E2-type pathway was energetically favored in the cyclohexenone substrate. These energetic barriers were consistent with their experiment findings and help explain why the DMSO oxygen is not transferred to the ring when starting with cyclohexenone.
In addition, the Jiao group found that in most cases when the cyclohexanone ring contained more than one substituent, it too produced the substituted phenol under the I2/DMSO reaction conditions. It is assumed that the extra substituents in part block the ability of DMSO to act as a nucleophile to displace iodide, thereby making the E2 reaction pathway competitive. These cases, where phenol is produced from multiply-substituted cyclohexanones, indicate the first reported instances of metal-free transformation of cyclohexanone to a phenol.
In summary, the Jiao group has found a metal-free, mild and simple method to make catechols and highly-substituted phenols from cyclohexanones. This route is attractive in that the reagents and solvent are inexpensive and readily available, and they are considered practical and green alternatives to other methods.
Debra D. Dolliver, Ph.D.
References
(1) Liang, Y.-F.; Li, X.; Wang, X.; Zou, M.; Tang, C.; Liang, Y.; Song, S.; Jiao, N. Journal of the American Chemical Society 2016, 138, 12271.
(2) Li, W.; Xie, D.; Frost, J. W. Journal of the American Chemical Society 2005, 127, 2874.
(3) Diao, T.; Pun, D.; Stahl, S. S. Journal of the American Chemical Society 2013, 135, 8205.
(4) Pun, D.; Diao, T.; Stahl, S. S. Journal of the American Chemical Society 2013, 135, 8213.