Copper-Catalyzed Synthesis of Multisubstituted Indoles in DMSO
The indole subunit is widely seen in many pharmaceutically and biologically active compounds. Its importance, therefore, has spurred the development of numerous methods to synthesize this subunit. Additionally, new methods continue to be developed to overcome some of the deficiencies or limitations of past methods such as harsh conditions, exotic starting materials, and expensive or toxic materials, etc.
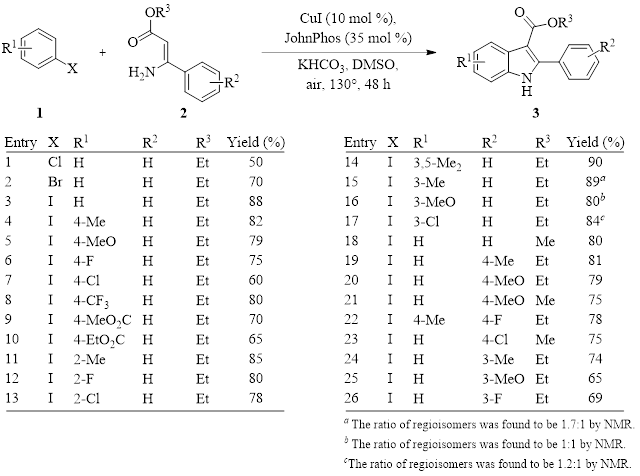
The Peng/Chen group has recently published a method that allows for the modular assembly of highly-functionalized indoles 3 (Table 1) through two tandem copper-catalyzed couplings (an Ullmann-type coupling and a cross-dehydrogenative coupling).1 These tandem reactions offered the best yield when run in DMSO, and this work represents another example where copper-catalyzed reactions produce superior results in DMSO. (For other examples of copper-catalyzed reactions run in DMSO, see recent Synthesis Corner posts from May 30, 2018 and January 18, 2018.)
Optimization experiments indicate that the best yields were afforded with the use of potassium bicarbonate as the base (after screening seven bases), JohnPhos as the ligand (after screening six ligands), and CuI as the source of copper [after screening seven Cu(I) and Cu(II) salts].
These researchers then investigated the scope of these reactions by changing the character of the arylhalide electophile 1 and the nature of the 3-aminocinnamic ester 2. As with many metal-catalyzed arylhalide-amine coupling reactions, the aryliodide outperforms either the bromide or chloride (Table 1, entries 1–3). All subsequent reactions were therefore run with the iodo-substituted aromatic compound.
As can be seen, all starting materials provide good yields, and there is little effect from electron-donating or -withdrawing groups or position of the substituent. (One reaction is not shown in Table 1— the reaction of 1-iodonaphthalene with ethyl-3-aminocinnamate. This reaction also provides a good yield of 81%.) Although this reaction must be run at a fairly elevated temperature (130°) to accomplish the tandem coupling reactions, it is tolerant to air and only requires standard laboratory equipment and procedures.
Table 1: Optimized Conditions and Scope of the Reaction

In summary, the Peng/Chen group has demonstrated the use of two tandem copper-catalyzed coupling reactions of aryl iodides and 3-aminocinnamic esters to yield highly functionalized indoles. This modular assembly, using inexpensive copper catalysis, is a valuable addition to the synthesis of the important indole structural motif.
Debra D. Dolliver, Ph.D.
References:
1 Li, Y.; Peng, J.; Chen, X.; Mo, B.; Li, X.; Sun, P.; Chen, C. J. Org. Chem. 2018, 83, 5288.







