N,Nʹ-Diaryl-o-phenylenediamines are precursors to many N-heterocyclic carbenes and are substructures of many medicinal substances, yet there are few routes to synthesize these compounds. This makes the metal-free method recently published by the Pan group particularly attractive.1 The method described by Pan’s group is simple, uses easily-acquired starting materials, and, with the use of the DMSO/I2 catalyst, is relatively environmentally friendly.
The DMSO/I2 pair is becoming more and more common in synthetic schemes. (For recent examples, see Synthesis Corner posts from Oct. 1, 2019 and July 28, 2017.) These generally proceed through a mild iodination functionalization step followed by a Kornblum oxidation. DMSO acts as an oxidant for the iodized substrate and then acts as an oxidant to return iodide to iodine so that it can reenter the catalytic cycle.
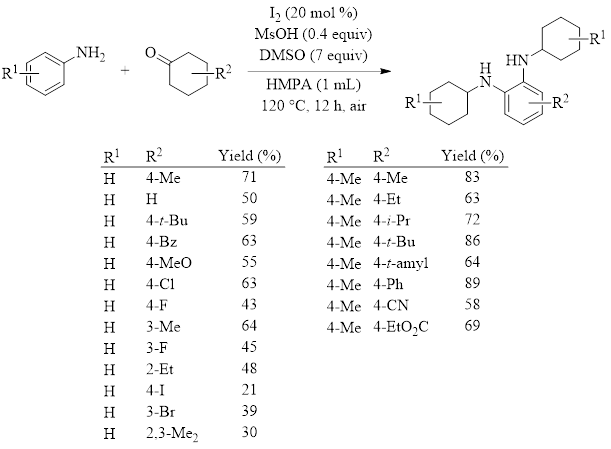
The Pan group optimized the reaction conditions for the synthesis of phenylenediamines (equation in Table 1), and they tested the substrate scope with various cyclohexanones and anilines (Table 1). As can be seen, the yields are good except in a few cases.
Table 1: Synthesis of N,Nʹ-diaryl-o-phenylenediamines

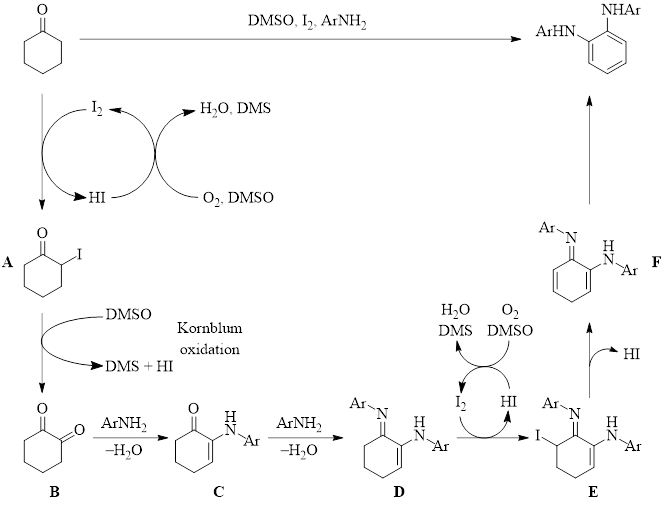
Mechanistic studies indicated that the reaction proceeds in the presence of TEMPO, ruling out radical intermediates. They also found that starting with 1,2-cyclohexadione with the same reaction conditions also yields the phenylenediamine production, indicating that the diketone is a likely intermediate. Additionally, the enamine C (Scheme 1) intermediate was also isolated from the reaction when using a different solvent. With this information in hand, the mechanism shown in Scheme 1 was proposed.
Scheme 1: Proposed Mechanism

In this reaction pathway, the ketone undergoes α-iodination to yield the iodinated ketone A. Ketone A then undergoes a Kornblum oxidation by DMSO to produce diketone B. Diketone B reacts with aniline to produce enamine C. Another equivalent of the aniline converts ketone-enamine C to imine-enamine D. A second α-iodination of intermediate D produces the α-iodoimine E. Loss of HI from intermediate E begins the aromatization process, proceeding through intermediate F before forming the product.
This reaction illustrates another advance of the DMSO/I2 catalytic system. This reaction results in the synthesis of phenylenediamines from a relatively simple set up using readily-available cyclohexanones and anilines. Therefore, it is likely to find use in the synthesis of these valuable compounds.
Debra D. Dolliver, Ph.D.
References:
1 Xiong, M.; Gao, Z.; Liang, X.; Cai, P.; Zhu, H.; Pan, Y. Chemical Communications 2018, 54, 9679.
Contact Gaylord Chemical
We pride ourselves in delivering quality products, technical support and world-class customer service. Gaylord Chemical is an on-purpose manufacturer of DMS and DMSO. We are focused on these products and the customers that use them.
To purchase chemicals from Gaylord Chemical for your business, locate a distributor in your country. For research or quality commercial use, request a sample.