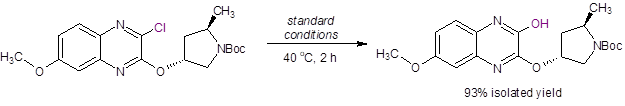Direct Conversion of Haloarenes to Phenols with Acetohydroxamic acid in DMSO
Phenols are widely used in synthetic schemes, and they occur in many natural products and medicinally-useful compounds. Nevertheless, there are only a limited number of methods available to produce the phenolic group from readily available haloarenes. The common methods to convert haloarenes to phenols have a variety of drawbacks, including high temperatures, strongly basic medium, sensitivity to oxygen, and the necessity of expensive/toxic transition metal catalysts and/or ligands.1
Fier and Maloney, however, have recently developed a method whereby acetohydroxamic acid in DMSO acts as a nucleophilic surrogate for hydroxide ion and readily converts electron-deficient haloarenes to phenols.2 Their method is relatively simple, mild, and high-yielding. It requires no expensive catalysts or exotic conditions.
The reaction appears to proceed through a two-step, one-pot pathway: nucleophilic aromatic substitution (SNAr) followed by an in-situ Lossen rearrangement (Equation 1).
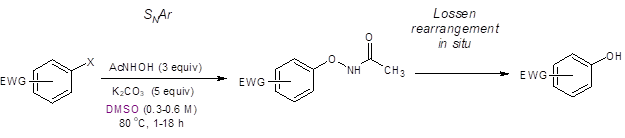
Equation 1: Optimized conditions for the synthesis of phenols from arylhalides, acetohydroxamic acid and mild base in DMSO.
The optimized conditions shown in Equation 1 offered excellent isolated yields for a variety of electron deficient aryl halides (Scheme 1), even at the 2.0-5.0 mmol scale.

Scheme 1: Isolated yields of the corresponding phenol 2 when starting with a variety of electron deficient haloarenes (1a-1l).
Additionally, the reaction worked well with a variety of nitrogen-containing haloarenes (Scheme 2). These reactions also produced excellent isolated yields, again at the 2.0-5.0 mmol scale.
Scheme 2: Isolated yields of the corresponding phenol 4 when starting with a variety of electron deficient nitrogen-containing haloarenes (3a-3f).
These mild reaction conditions allowed for use of this hydroxylation reaction with more complex molecules containing sensitive moieties. To demonstrate this reaction’s utility, Fier and Maloney performed the hydroxylation reaction on densely functionalized pharmaceutical molecules.
As illustrated in Equation 2 they successfully performed the reaction on a compound containing an ester, a primary aniline, an aryl chloride, and an ortho-acetamide group. Of note, when attempting the SNAr reaction on this compound under strongly basic conditions, reaction occurred at the acetamide group. This unwanted side reaction is totally avoided when performing the reaction under the conditions described in the Fier and Maloney paper.
Equation 2: Successful hydroxylation reaction on a heavily functionalized compound.
Additionally, they applied these reaction conditions to an intermediate in the synthesis of Grazoprevir (MK-5172), a selective NS3/4a protease inhibitor for the treatment of HCV (Equation 3). Again, excellent isolated yield was achieved with no epimerization at the stereocenters. In fact, this product was directly isolated after the addition of water and dilute HCl.
Equation 3: Successful hydroxylation reaction of an intermediate in the synthesis of Grazoprevir
In conclusion, these investigators have developed a high-yielding and mild method to convert election-deficient haloarenes into phenols. This reaction tolerates a variety of functional groups. In addition, no expensive, exotic, toxic or strongly basic reagents are required.
Debra D. Dolliver, Ph.D.
References
- For recent examples, see: (a) Enthaler, S.; Company, A., Palladium-catalysed hydroxylation and alkoxylation. Chemical Society Reviews 2011, 40 (10), 4912-4924; (b) Cheung, C. W.; Buchwald, S. L., Palladium-Catalyzed Hydroxylation of Aryl and Heteroaryl Halides Enabled by the Use of a Palladacycle Precatalyst. The Journal of Organic Chemistry 2014, 79 (11), 5351-5358; (c) Song, G.-L.; Zhang, Z.; Da, Y.-X.; Wang, X.-C., Copper and l-sodium ascorbate catalyzed hydroxylation and aryloxylation of aryl halides. Tetrahedron 2015, 71 (46), 8823-8829.
- Fier, P. S.; Maloney, K. M., Direct Conversion of Haloarenes to Phenols under Mild, Transition-Metal-Free Conditions. Organic Letters 2016, 18 (9), 2244-2247.