Amino and nitro-substituted naphthalenes are important structural units in pharmaceutical materials. Recently, the Verma group reported the first synthesis of nitronaphthylamines through a one pot reaction that involves an aza-Henry reaction followed by an annulation.1 This synthesis uses nitromethane in KOH/DMSO. This method can be extended to incorporate acetonitrile or benzophenone in place of nitromethane (Figure 1).
Synthesis of Nitronaphthylamines using KOH/DMSO
This reaction requires no metal catalyst and uses simple, readily available reagents. It is tolerant of a variety of functional groups on the alkynylnitrile species at both the R1 and the R2 positions.
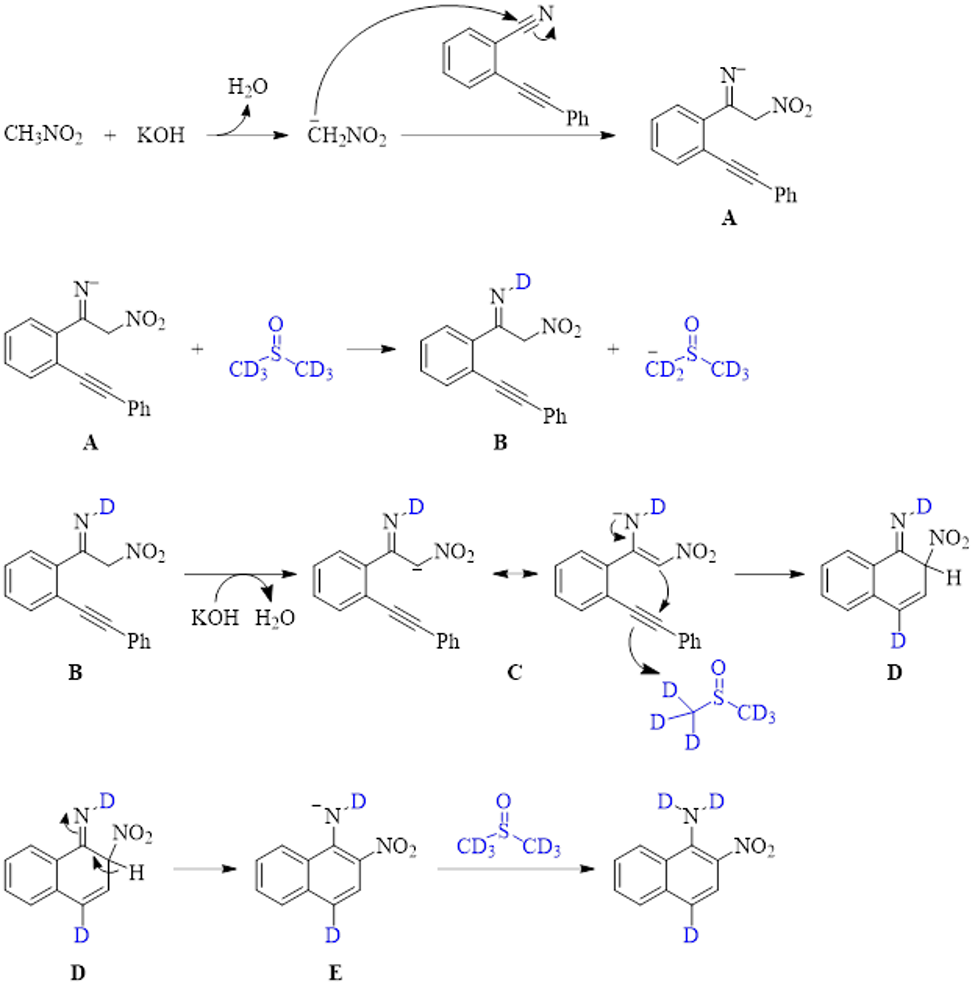
Mechanistic studies indicated that the reaction proceeds in the presence of a radical inhibitor, and reaction in the presence of deuterated DMSO resulted in 100% deuteration at the ring position para to the amino group and 70% deuteration of the amine group. With this information, the mechanism shown in Scheme 1 was proposed to be the dominant pathway.
In this mechanism, the first step is an aza-Henry addition where the deprotonated nitromethane attacks the nitrile to produce intermediate species A. Anion A can either be deuterated by DMSO-d6 to produce imine B or protonated by another molecule of nitromethane, accounting for the 30% portion of the final product where the amine functionality contains protons instead of deuterium atoms. Species B can be deprotonated by an equivalent of base, and the intermediate enamine C can cyclize and react with a molecule of DMSO-d6, generating intermediate D. The elimination of a proton from the ring producing intermediate E and the deuteration or protonation of the resultant nitrogen anion results in the final product.
In summary, this reaction is the first reported route to polyfunctional nitronaphthylamines. The reaction is robust in its tolerance of functional groups and has been demonstrated to work in molecules containing labile functionality. The reaction does not require a metal catalyst, and all the reagents are commercially readily available.
Debra D. Dolliver, Ph.D.
- Verma, S.; Kumar, M.; Verma, A. K., Aza-Henry Reaction: Synthesis of Nitronaphthylamines from 2-(Alkynyl)benzonitriles. Organic Letters 2020, 22 (1), 130-134.