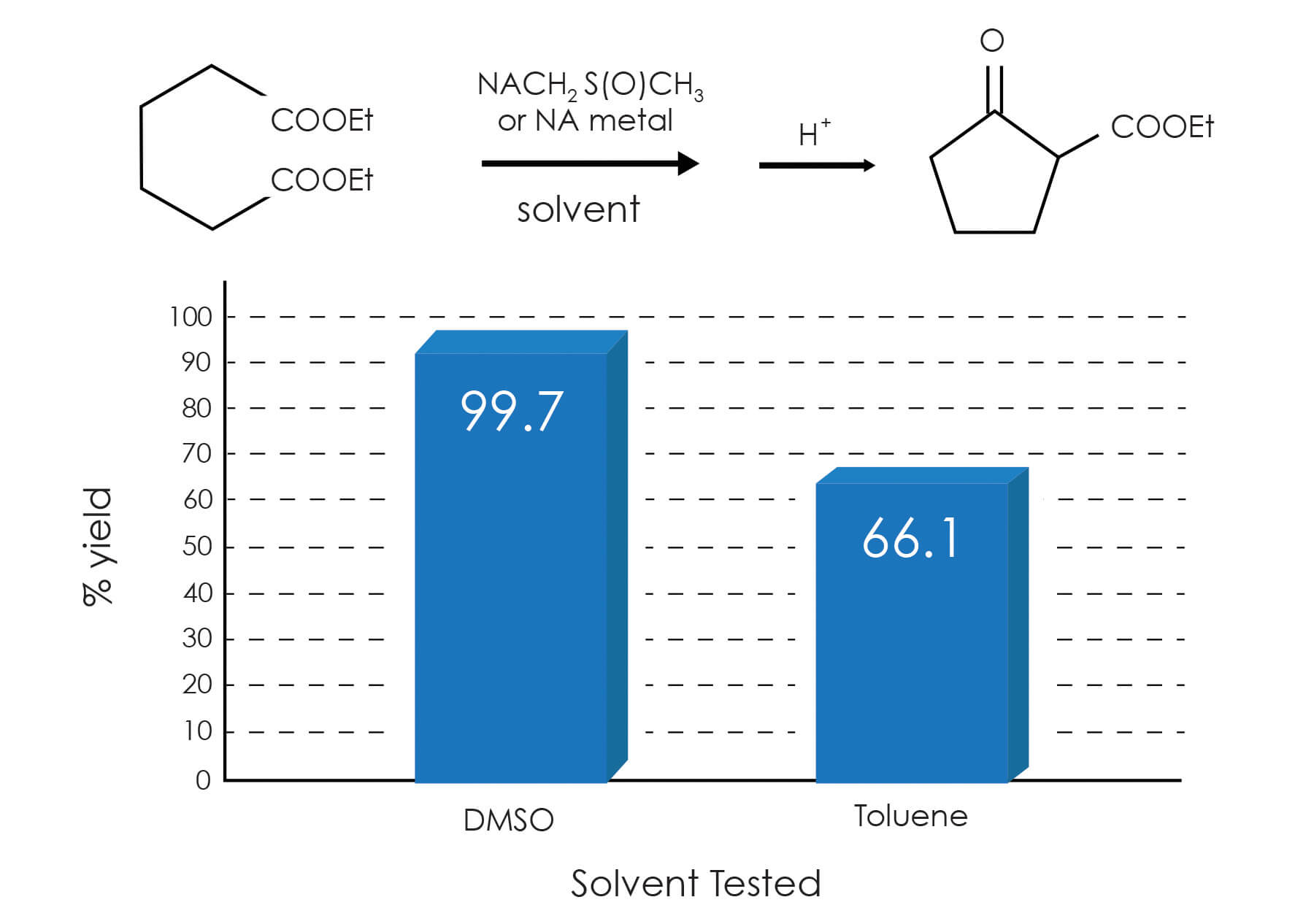
The Dieckmann Cyclization is one of the important reactions that we have evaluated. Not only is this reaction of a higher order of importance as a carbon-carbon bond-forming reaction, but it is one of the best ways to form 5-membered carbocyclic rings. This reaction involves the base-catalyzed cyclization of dicarboxylic esters to give ß-ketoesters, the intramolecular equivalent of the Claisen condensation. We have investigated the yields obtained using DMSO with dimsyl ion as the base versus a traditional method employing toluene and sodium metal. The graph below displays one of the examples where the usage of DMSO provides significantly higher yields: