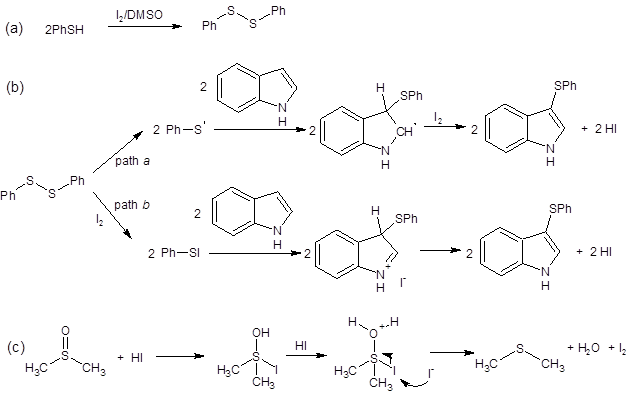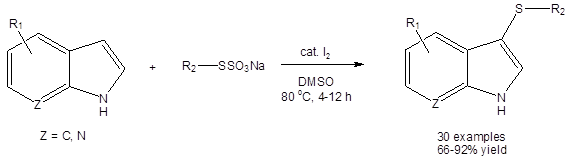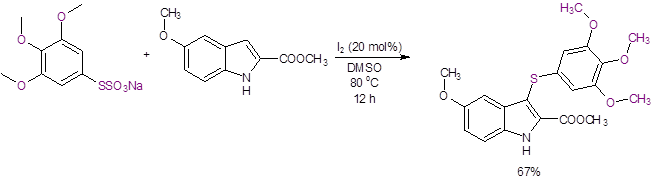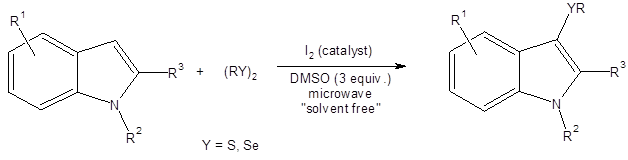
Equation 1: Chalcogenylation at the 3 position of indoles using diselenides of disulfides as the chalcogen source.
In early 2016, there are two new reports where the DMSO/I2 catalytic system has been used to produce an electrophilic RS-I species which is then available for an electrophilic substitution reaction.3 Both of these papers show that this catalyst system provides green, mild, metal-free, and operationally simple conditions for organothiolation at the 3 position of indoles.
3-Thioindoles have shown potential for use in therapies for the treatment of HIV,4 cancer,5 obesity,6 and heart disease.7 Therefore, routes to thiolate the indole core using readily available sulfur sources without the need for a metal catalyst are very attractive.
Synthesis using Thiols as the sulfur source:
Shen, et al., have shown that the DMSO/I2 system can efficiently catalyze the organothiolation of the 3-position of various indoles with thiols acting as the sulfur source (Equation 2).3a
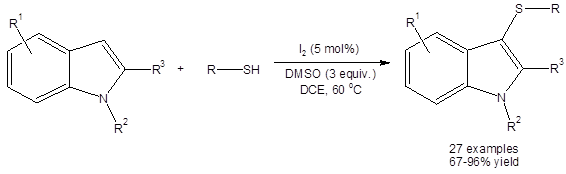
Equation 2: DMSO/I2 catalyzed synthesis of 3-thioindoles using thiols as the sulfur source.
All yields were excellent (except when R3 was an ethyl ester group, 67%). Interestingly, yields were high (>89%) when R3 was a phenyl ring. Electron withdrawing and electron donating group substituents were well tolerated on both the aromatic ring of the aryl thiol and on the six membered ring of the indole.
Shen’s group reported that two mechanistic pathways may be at play in this transformation. As seen in Scheme 1, two pathways have been proposed (path a and path b). Path a involves formation of a thio radical. This mechanism is supported by the fact that adding the radical-trapping reagent TEMPO reduced the yield of the model reaction from 97% to 30%. The researchers propose a second reaction pathway where electrophilic PhS-I is formed in situ (path b). This route is supported by literature1-2, 8 and by the fact that the reaction still proceeds when the radical pathway is shut down by TEMPO.
Regardless of the pathway, they both depend on DMSO oxidizing I– to regenerate the I2 catalyst (equation c in Scheme 1).
Scheme 1: Proposed mechanistic pathways when using thiols as the sulfur source
Synthesis using Bunte Salts as the sulfur source:
Luo, et al., used Bunte salts (RSSO3Na) as the sulfur source in their synthesis of 3-thioindoles (Equation 3).3b
Equation 3: DMSO/I2 catalyzed synthesis of 3-thioindoles using Bunte salts as the sulfur source.
Bunte salts are readily prepared from sodium thiosulfate and various organohalides.9 They are stable, easy to handle and odorless. The reaction gave excellent yields in all cases but one where the indole core was substituted at the 2-position with an ester group (66%).
The authors proposed the mechanistic pathway for the reaction shown in Scheme 2. Here, the Bunte salt reacts with I2 to produce the electrophilic RS-I. This undergoes electrophilic substitution with the indole ring system at the 3-position. DMSO oxidizes the resultant iodide ion back to I2. DMSO acts as both the solvent and as a mild in situ oxidant in this reaction and allows the reaction to be run with only catalytic amounts of iodine.
Scheme 2: Proposed mechanism for the organothiolation using Bunte salts.
Using this reaction, Luo et al. synthesized a potent inhibitor of tubulin polymerization that shows excellent antitumor activity with a good yield of 67% (Equation 4). Prior synthesis of this 3-thioindole by a traditional method had only resulted in a very low yield (4%).10
Equation 4: Synthesis of a 3-thioindole with potent antitumor properties.
In particular, the Luo reaction is attractive as it uses a non-metal catalyst, an oxidant/solvent with low toxicity (DMSO), and inexpensive and readily-available reagents. In addition, it is operationally simple to perform.
Debra D. Dolliver, Ph.D.
References
- Azeredo, J. B.; Godoi, M.; Martins, G. M.; Silveira, C. C.; Braga, A. L., A Solvent- and Metal-Free Synthesis of 3-Chalcogenyl-indoles Employing DMSO/I2 as an Eco-friendly Catalytic Oxidation System. J. Org. Chem. 2014, 79 (9), 4125-4130.
- Parumala, S. K. R.; Peddinti, R. K., Iodine catalyzed cross-dehydrogenative C-S coupling by C(sp2)-H bond activation: direct access to aryl sulfides from aryl thiols. Green Chem. 2015, 17 (7), 4068-4072.
- (a) Yi, S.; Li, M.; Mo, W.; Hu, X.; Hu, B.; Sun, N.; Jin, L.; Shen, Z., Metal-free, iodine-catalyzed regioselective sulfenylation of indoles with thiols. Tetrahedron Lett. 2016, 57 (17), 1912-1916; (b) Qi, H.; Zhang, T.; Wan, K.; Luo, M., Catalytic Synthesis of 3-Thioindoles Using Bunte Salts as Sulfur Sources under Metal-Free Conditions. J. Org. Chem. 2016, 81 (10), 4262-4268.
- Ragno, R.; Coluccia, A.; La Regina, G.; De Martino, G.; Piscitelli, F.; Lavecchia, A.; Novellino, E.; Bergamini, A.; Ciaprini, C.; Sinistro, A.; Maga, G.; Crespan, E.; Artico, M.; Silvestri, R., Design, Molecular Modeling, Synthesis, and Anti-HIV-1 Activity of New Indolyl Aryl Sulfones. Novel Derivatives of the Indole-2-carboxamide. J. Med. Chem. 2006, 49 (11), 3172-3184.
- Cianchi, F.; Cortesini, C.; Magnelli, L.; Fanti, E.; Papucci, L.; Schiavone, N.; Messerini, L.; Vannacci, A.; Capaccioli, S.; Perna, F.; Lulli, M.; Fabbroni, V.; Perigli, G.; Bechi, P.; Masini, E., Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol. Cancer Ther. 2006, 5 (11), 2716-2726.
- Ramakrishna, V. S. N.; Shirsath, V. S.; Kambhampati, R. S.; Vishwakarma, S.; Kandikere, N. V.; Kota, S.; Jasti, V. Preparation of benzenesulfonyl indole derivatives as functional 5-HT6 ligands for treatment of CNS disorder. WO2007020653A1, 2007.
- Funk, C. D., Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat. Rev. Drug Discovery 2005, 4 (8), 664-672.
- Zhang, H.; Bao, X.; Song, Y.; Qu, J.; Wang, B., Iodine-catalyzed versatile sulfenylation of indoles with thiophenols: controllable synthesis of mono- and bis-arylthioindoles. Tetrahedron 2015, 71 (47), 8885-8891.
- Reeves, J. T.; Camara, K.; Han, Z. S.; Xu, Y.; Lee, H.; Busacca, C. A.; Senanayake, C. H., The Reaction of Grignard Reagents with Bunte Salts: A Thiol-Free Synthesis of Sulfides. Org. Lett. 2014, 16 (4), 1196-1199.
- Hamel, E.; Silvestri, R.; Brancale, A. Arylthioindole tubulin polymerization inhibitors and their preparation, pharmaceutical compositions, and methods of treating or preventing cancer using them. WO2006041961A1, 2006.